Immunovant Corporate Update April 21, 2025 Exhibit 99.2

2 Forward-Looking Statements Roivant Forward-Looking Statements This presentation includes forward-looking statements that are subject to substantial risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements. All statements other than statements of historical facts contained in this presentation, including statements regarding our future results of operations and financial position, business strategy, potential uses of cash and capital allocation, research and development plans, profitability, the anticipated timing, costs, design, conduct and results of our ongoing and planned preclinical studies and clinical trials for our products and product candidates, and any commercial potential of our products and product candidates are forward- looking statements. These forward-looking statements are based upon the current expectations and beliefs of our management as of the date of this presentation and are subject to certain risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. Although we believe that our plans, intentions, expectations and strategies as reflected in or suggested by those forward-looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved. Furthermore, actual results may differ materially from those described in the forward-looking statements. These forward-looking statements may be affected by a number of risks, uncertainties and assumptions, including, but not limited to, those risks set forth in the sections captioned “Risk Factors” and “Forward- Looking Statements” of our filings with the U.S. Securities and Exchange Commission, available at www.sec.gov and investor.roivant.com. We operate in a very competitive and rapidly changing environment in which new risks emerge from time to time. These forward-looking statements are based upon the current expectations and beliefs of our management as of the date of this presentation, and are subject to certain risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. Except as required by applicable law, we assume no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise. Immunovant Forward-Looking Statements This presentation contains forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. The use of words such as "can," “may,” “might,” “will,” “would,” “should,” “expect,” “believe,” “estimate,” “design,” “plan,” "intend," "anticipate," and other similar expressions are intended to identify forward-looking statements. Such forward looking statements include Immunovant’s expectations regarding the goals of its clinical development programs, including the efficacy, safety, and clinical success of batoclimab in Immunovant’s myasthenia gravis (MG) and chronic inflammatory demyelinating polyneuropathy (CIDP) programs; belief in the performance, magnitude of benefit, or best-in-class results shown with batoclimab relative to therapies evaluated in other trials; plans and expectations for a pivotal trial of IMVT-1402 in MG, including the timing thereof; expectations regarding the potential for IMVT-1402 to meet or exceed the results observed in studies of batoclimab; beliefs regarding the best-in-class potential of IMVT-1402; and the anticipated benefits of Immunovant’s strategic reprioritization from batoclimab to IMVT-1402. All forward-looking statements are based on estimates and assumptions by Immunovant’s management that, although Immunovant believes to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that Immunovant expected. Such risks and uncertainties include, among others: initial results or other preliminary analyses or results of early clinical trials may not be predictive of final trial results or of the results of later clinical trials; results of animal studies may not be predictive of results in humans; the timing and availability of data from clinical trials; the timing of discussions with regulatory agencies, as well as regulatory submissions and potential approvals; the continued development of Immunovant’s product candidates, including the timing of the commencement of additional clinical trials; Immunovant’s scientific approach, clinical trial design, indication selection, and general development progress; future clinical trials may not confirm any safety, potency, or other product characteristics described or assumed in this presentation; any product candidate that Immunovant develops may not progress through clinical development or receive required regulatory approvals within expected timelines or at all; Immunovant’s product candidates may not be beneficial to patients, or even if approved by regulatory authorities, successfully commercialized; the effect of global factors such as geopolitical tensions and adverse macroeconomic conditions on Immunovant’s business operations and supply chains, including its clinical development plans and timelines; Immunovant’s business is heavily dependent on the successful development, regulatory approval and commercialization of batoclimab and IMVT-1402; Immunovant is in various stages of clinical development for IMVT-1402 and batoclimab; Immunovant’s intellectual property position; and Immunovant will require additional capital to fund its operations and advance IMVT-1402 and batoclimab through clinical development. These and other risks and uncertainties are more fully described in Immunovant’s periodic and other reports filed with the Securities and Exchange Commission (SEC), including in the section titled “Risk Factors” in Immunovant’s most recent Quarterly Report on Form 10-Q for the quarter ended December 31, 2024, filed with the SEC on February 6, 2025, and Immunovant’s subsequent filings with the SEC. Any forward-looking statement speaks only as of the date on which it was made. Immunovant undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products. Disclaimer This presentation is intended for the investor community only; it is not intended to promote the product candidates referenced herein or otherwise influence healthcare prescribing decisions. For investor audiences only
• Eric Venker, MD appointed CEO (President & COO of Roivant) • Tiago Girao appointed CFO (Former Telavant CFO) • Pete Salzmann, MD to remain as advisor following retirement 3 • Rapid clinical execution in potentially registrational studies for IMVT-1402 • Commercial planning for multiple potential upcoming 1402 launches • Careful allocation of resources and capital Strategic Focus (Next Leg)Management Transition • Sjögren’s Disease (SjD): IND cleared, potentially registrational trial starts Summer 2025 (potential for best-in-class profile) • Cutaneous Lupus Erythematosus (CLE): Concept study initiated (potential first-in-class; early positive data) • IMVT focused on executing in 6 announced indications currently underway (including SjD and CLE) IMVT-1402 Pipeline Update Immunovant: Driving the Next Phase of Growth with Roivant For investor audiences only Current cash balance provides runway for announced indications into Graves’ readout expected in 2027 with further broadening of indication set to be closely evaluated

4 Dr. Venker has served on the Board of Directors of Immunovant since 2020 and is also the President and Chief Operating Officer of Roivant. He will continue to hold these positions. Dr. Venker joined Roivant in 2014 and has since served in various roles of increasing responsibility, including Chief of Staff to the CEO. Prior to joining Roivant, Dr. Venker was a physician at New York Presbyterian Hospital, Columbia University Medical Center, where he trained in internal medicine. While there, Dr. Venker led operational initiatives to improve efficiencies across a large hospital system with $5 billion in annual revenue. Earlier in his career, Dr. Venker was a Clinical Pharmacist at Yale-New Haven Hospital. Dr. Venker received his M.D. from Yale School of Medicine and his Pharm.D. from St. Louis College of Pharmacy. Dr. Venker bring overs two decades of clinical practice and biopharma operating experience to the company Introducing Eric Venker, MD as New CEO of Immunovant For investor audiences only
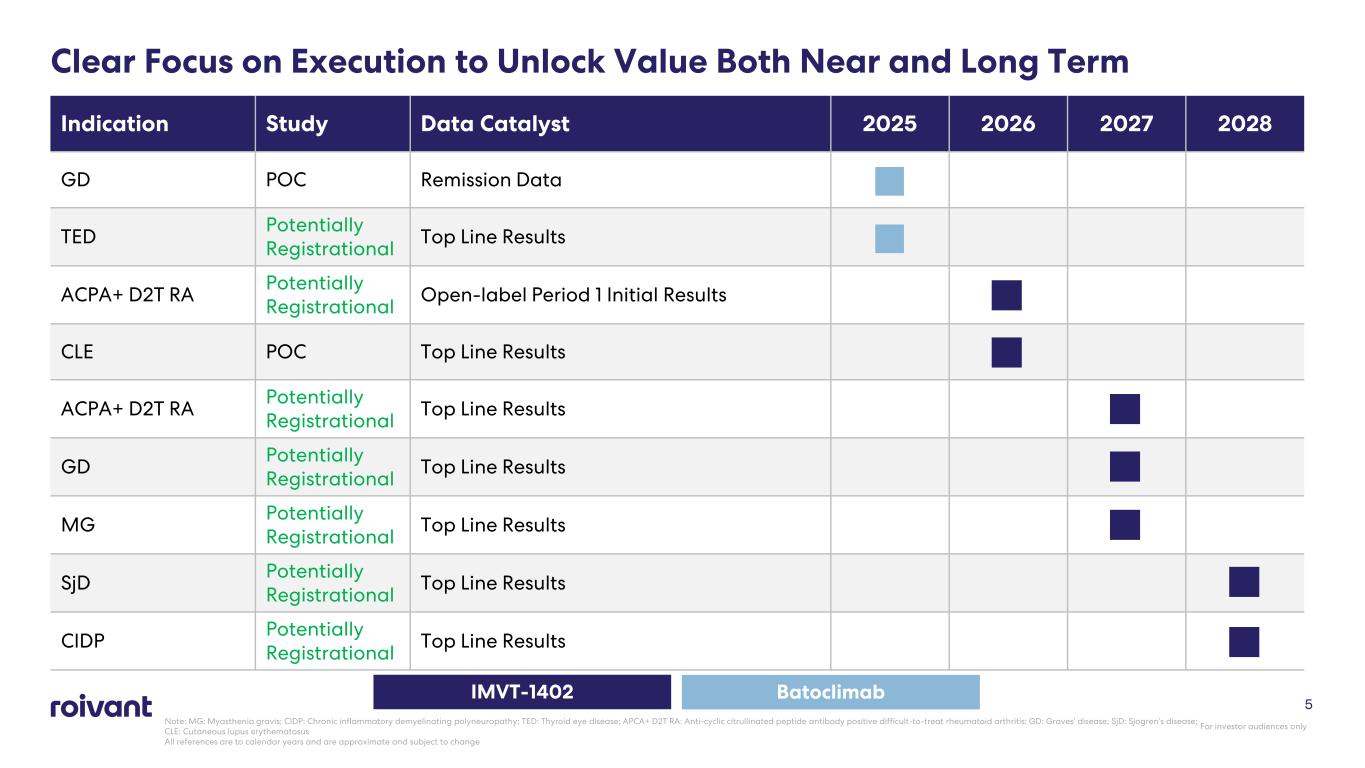
Clear Focus on Execution to Unlock Value Both Near and Long Term Indication Study Data Catalyst 2025 2026 2027 2028 GD POC Remission Data TED Potentially Registrational Top Line Results ACPA+ D2T RA Potentially Registrational Open-label Period 1 Initial Results CLE POC Top Line Results ACPA+ D2T RA Potentially Registrational Top Line Results GD Potentially Registrational Top Line Results MG Potentially Registrational Top Line Results SjD Potentially Registrational Top Line Results CIDP Potentially Registrational Top Line Results IMVT-1402 Batoclimab 5 For investor audiences only Note: MG: Myasthenia gravis; CIDP: Chronic inflammatory demyelinating polyneuropathy; TED: Thyroid eye disease; APCA+ D2T RA: Anti-cyclic citrullinated peptide antibody positive difficult-to-treat rheumatoid arthritis; GD: Graves’ disease; SjD: Sjogren’s disease; CLE: Cutaneous lupus erythematosus All references are to calendar years and are approximate and subject to change
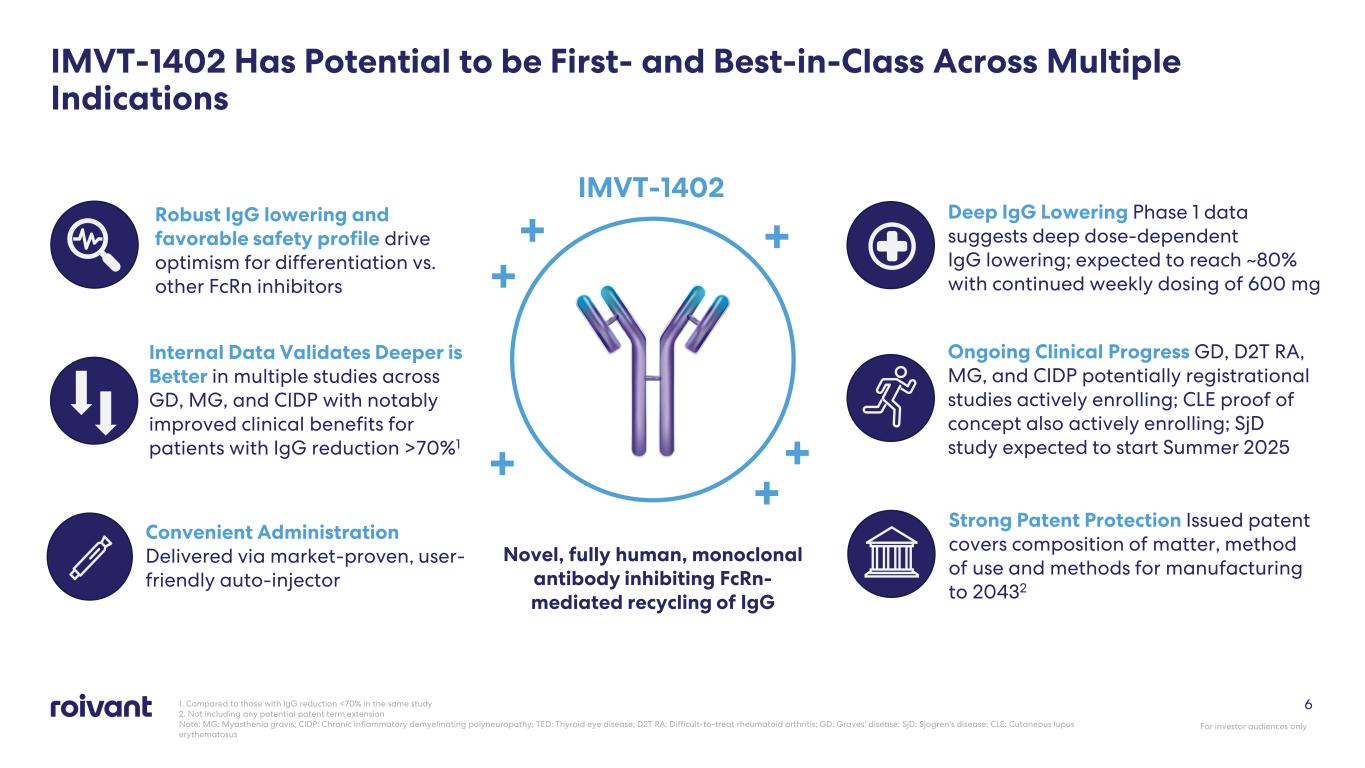
1. Compared to those with IgG reduction <70% in the same study 2. Not including any potential patent term extension Note: MG: Myasthenia gravis; CIDP: Chronic inflammatory demyelinating polyneuropathy; TED: Thyroid eye disease; D2T RA: Difficult-to-treat rheumatoid arthritis; GD: Graves’ disease; SjD: Sjogren’s disease; CLE: Cutaneous lupus erythematosus 6 IMVT-1402 Has Potential to be First- and Best-in-Class Across Multiple Indications Novel, fully human, monoclonal antibody inhibiting FcRn- mediated recycling of IgG + IMVT-1402 ++ + + + For investor audiences only Deep IgG Lowering Phase 1 data suggests deep dose-dependent IgG lowering; expected to reach ~80% with continued weekly dosing of 600 mg Ongoing Clinical Progress GD, D2T RA, MG, and CIDP potentially registrational studies actively enrolling; CLE proof of concept also actively enrolling; SjD study expected to start Summer 2025 Robust IgG lowering and favorable safety profile drive optimism for differentiation vs. other FcRn inhibitors Internal Data Validates Deeper is Better in multiple studies across GD, MG, and CIDP with notably improved clinical benefits for patients with IgG reduction >70%1 Convenient Administration Delivered via market-proven, user- friendly auto-injector Strong Patent Protection Issued patent covers composition of matter, method of use and methods for manufacturing to 20432
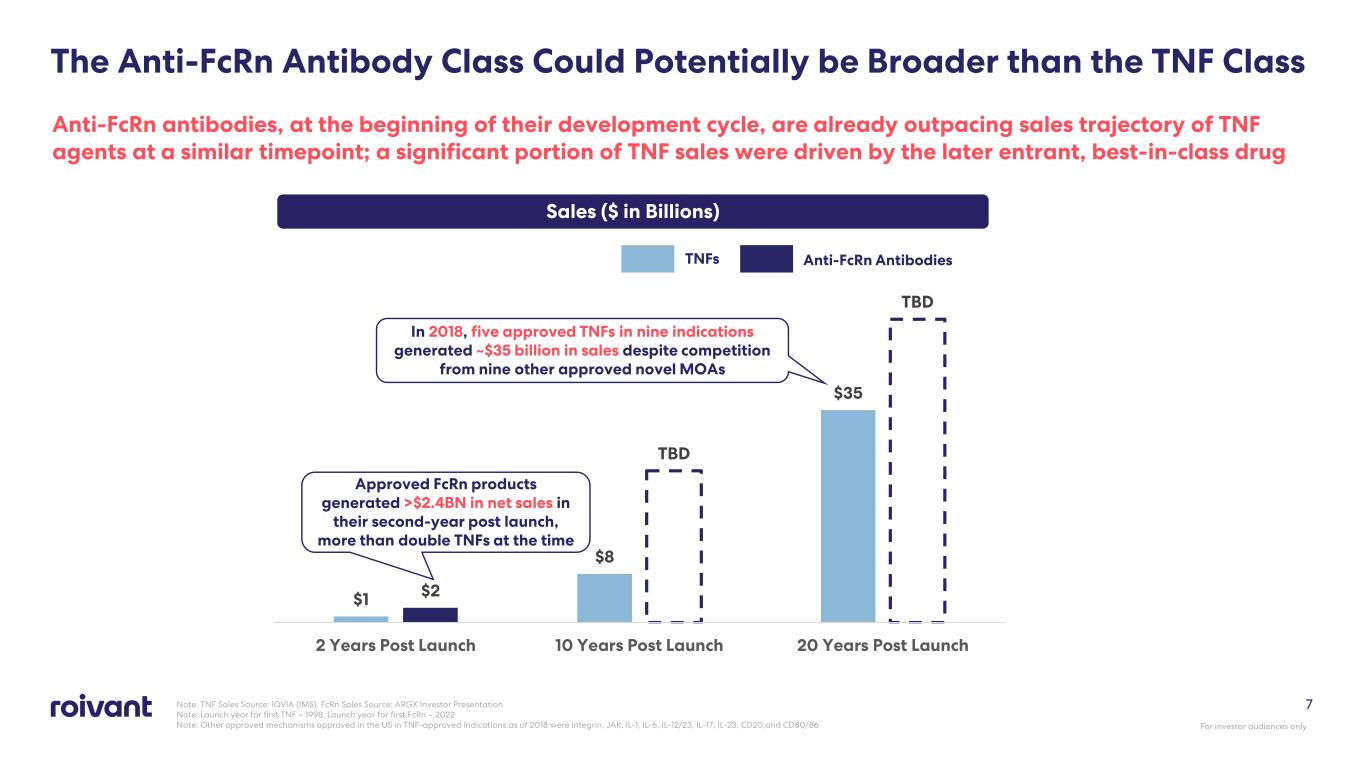
7 Anti-FcRn antibodies, at the beginning of their development cycle, are already outpacing sales trajectory of TNF agents at a similar timepoint; a significant portion of TNF sales were driven by the later entrant, best-in-class drug Note: TNF Sales Source: IQVIA (IMS). FcRn Sales Source: ARGX Investor Presentation Note: Launch year for first TNF – 1998, Launch year for first FcRn – 2022 Note: Other approved mechanisms approved in the US in TNF-approved indications as of 2018 were integrin, JAK, IL-1, IL-6, IL-12/23, IL-17, IL-23, CD20,and CD80/86 $1 $8 $35 $2 TBD TBD 2 Years Post Launch 10 Years Post Launch 20 Years Post Launch In 2018, five approved TNFs in nine indications generated ~$35 billion in sales despite competition from nine other approved novel MOAs Sales ($ in Billions) Approved FcRn products generated >$2.4BN in net sales in their second-year post launch, more than double TNFs at the time TNFs Anti-FcRn Antibodies The Anti-FcRn Antibody Class Could Potentially be Broader than the TNF Class For investor audiences only

Indication Strategy: Our FcRn Development Strategy is Designed for Maximum Commercial Potential, Leveraging 1402’s Potentially Best-in-Class Clinical Profile 8 Best-in-Class • Well-established markets with multiple competitors; potential to differentiate on efficacy • Example – MG and CIDP First-in-Class Best-in-Class • Expanding use of FcRn inhibitors to benefit greater number of patients with several new indications, with a potential efficacy advantage driven by deeper IgG reduction • Example – GD, D2T RA, Cutaneous Lupus Erythematosus (CLE) Nearly-First Best-in-Class • Close from a timing perspective to in-class competition, whilst maintaining potential for differentiated clinical profile driven by best-in-class IgG reductions • Example – Sjögren’s Disease (SjD) For investor audiences only IMVT-1402’s potentially differentiated product profile offers wide range of development opportunities
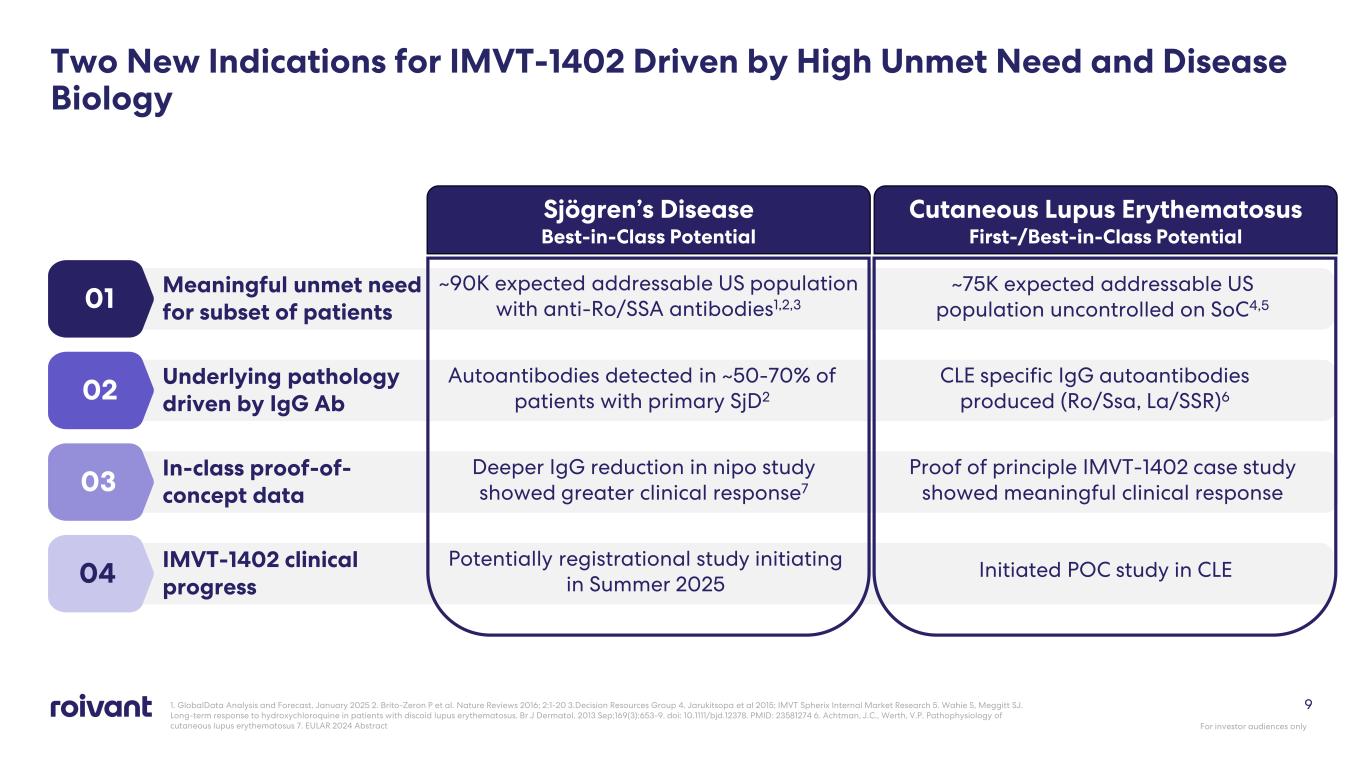
Two New Indications for IMVT-1402 Driven by High Unmet Need and Disease Biology Sjögren’s Disease Best-in-Class Potential Cutaneous Lupus Erythematosus First-/Best-in-Class Potential 01 Meaningful unmet need for subset of patients ~90K expected addressable US population with anti-Ro/SSA antibodies1,2,3 02 Underlying pathology driven by IgG Ab Autoantibodies detected in ~50-70% of patients with primary SjD2 CLE specific IgG autoantibodies produced (Ro/Ssa, La/SSR)6 04 IMVT-1402 clinical progress Potentially registrational study initiating in Summer 2025 Initiated POC study in CLE 03 In-class proof-of- concept data Deeper IgG reduction in nipo study showed greater clinical response7 Proof of principle IMVT-1402 case study showed meaningful clinical response 1. GlobalData Analysis and Forecast, January 2025 2. Brito-Zeron P et al. Nature Reviews 2016; 2:1-20 3.Decision Resources Group 4. Jarukitsopa et al 2015; IMVT Spherix Internal Market Research 5. Wahie S, Meggitt SJ. Long-term response to hydroxychloroquine in patients with discoid lupus erythematosus. Br J Dermatol. 2013 Sep;169(3):653-9. doi: 10.1111/bjd.12378. PMID: 23581274 6. Achtman, J.C., Werth, V.P. Pathophysiology of cutaneous lupus erythematosus 7. EULAR 2024 Abstract For investor audiences only 9 ~75K expected addressable US population uncontrolled on SoC4,5

Sjögren’s Disease Nearly-First- / Best-in-Class Opportunity
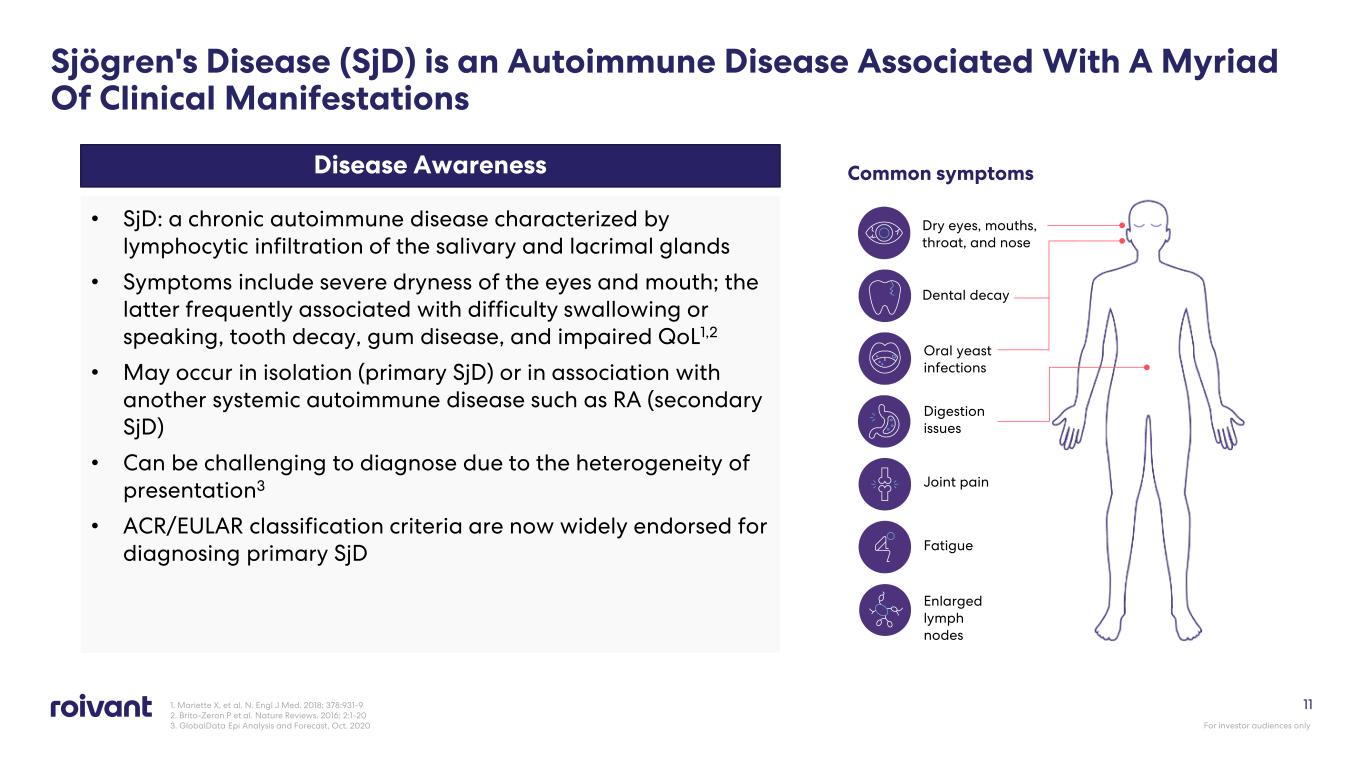
1. Mariette X, et al. N. Engl J Med. 2018; 378:931-9 2. Brito-Zeron P et al. Nature Reviews. 2016; 2:1-20 3. GlobalData Epi Analysis and Forecast, Oct. 2020 11 Disease Awareness • SjD: a chronic autoimmune disease characterized by lymphocytic infiltration of the salivary and lacrimal glands • Symptoms include severe dryness of the eyes and mouth; the latter frequently associated with difficulty swallowing or speaking, tooth decay, gum disease, and impaired QoL1,2 • May occur in isolation (primary SjD) or in association with another systemic autoimmune disease such as RA (secondary SjD) • Can be challenging to diagnose due to the heterogeneity of presentation3 • ACR/EULAR classification criteria are now widely endorsed for diagnosing primary SjD Common symptoms Dry eyes, mouths, throat, and nose Dental decay Oral yeast infections Digestion issues Joint pain Fatigue Enlarged lymph nodes Sjögren's Disease (SjD) is an Autoimmune Disease Associated With A Myriad Of Clinical Manifestations For investor audiences only
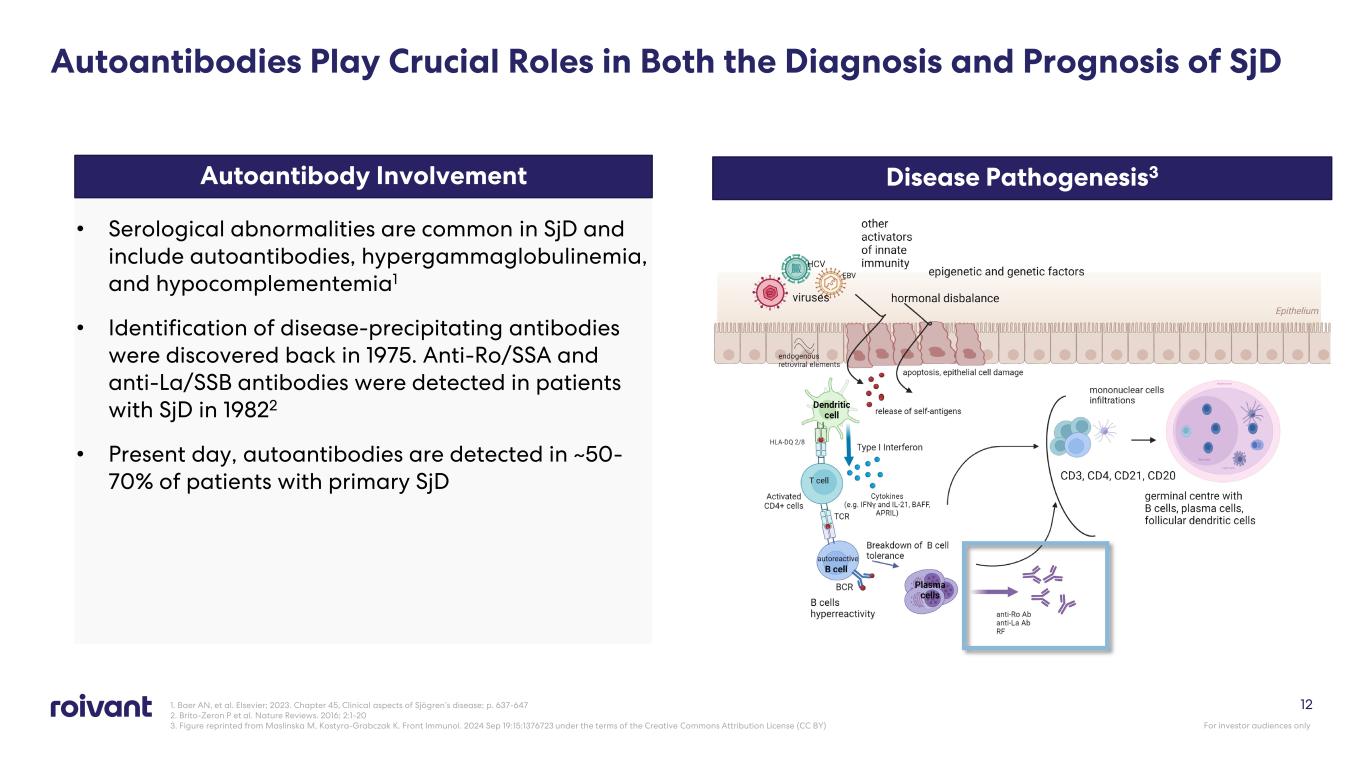
• Serological abnormalities are common in SjD and include autoantibodies, hypergammaglobulinemia, and hypocomplementemia1 • Identification of disease-precipitating antibodies were discovered back in 1975. Anti-Ro/SSA and anti-La/SSB antibodies were detected in patients with SjD in 19822 • Present day, autoantibodies are detected in ~50- 70% of patients with primary SjD 12 Autoantibody Involvement Disease Pathogenesis3 Autoantibodies Play Crucial Roles in Both the Diagnosis and Prognosis of SjD 1. Baer AN, et al. Elsevier; 2023. Chapter 45, Clinical aspects of Sjögren’s disease; p. 637-647 2. Brito-Zeron P et al. Nature Reviews. 2016; 2:1-20 3. Figure reprinted from Maslinska M, Kostyra-Grabczak K. Front Immunol. 2024 Sep 19:15:1376723 under the terms of the Creative Commons Attribution License (CC BY) For investor audiences only
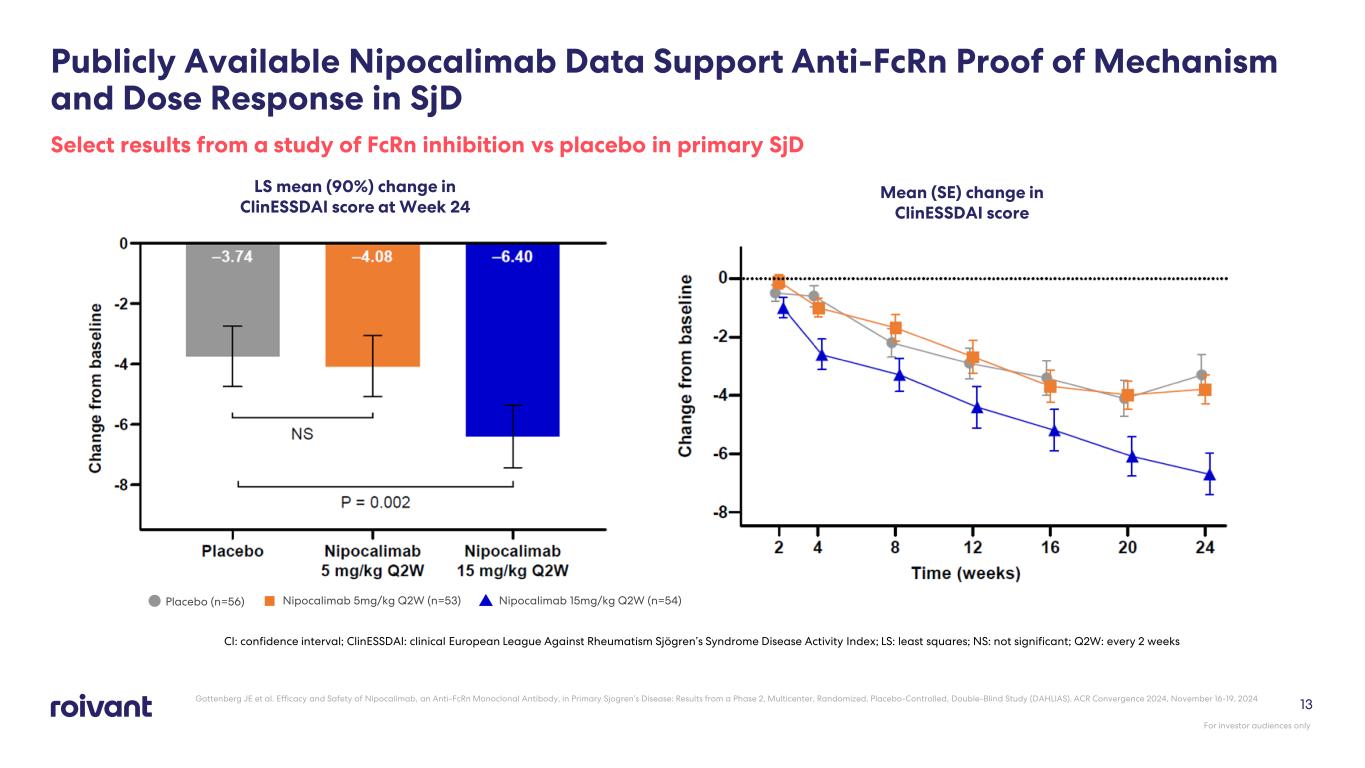
13 Publicly Available Nipocalimab Data Support Anti-FcRn Proof of Mechanism and Dose Response in SjD Select results from a study of FcRn inhibition vs placebo in primary SjD LS mean (90%) change in ClinESSDAI score at Week 24 Mean (SE) change in ClinESSDAI score CI: confidence interval; ClinESSDAI: clinical European League Against Rheumatism Sjögren’s Syndrome Disease Activity Index; LS: least squares; NS: not significant; Q2W: every 2 weeks Nipocalimab 15mg/kg Q2W (n=54)Placebo (n=56) Nipocalimab 5mg/kg Q2W (n=53) Gottenberg JE et al. Efficacy and Safety of Nipocalimab, an Anti-FcRn Monoclonal Antibody, in Primary Sjogren’s Disease: Results from a Phase 2, Multicenter, Randomized, Placebo-Controlled, Double-Blind Study (DAHLIAS). ACR Convergence 2024, November 16-19, 2024 For investor audiences only
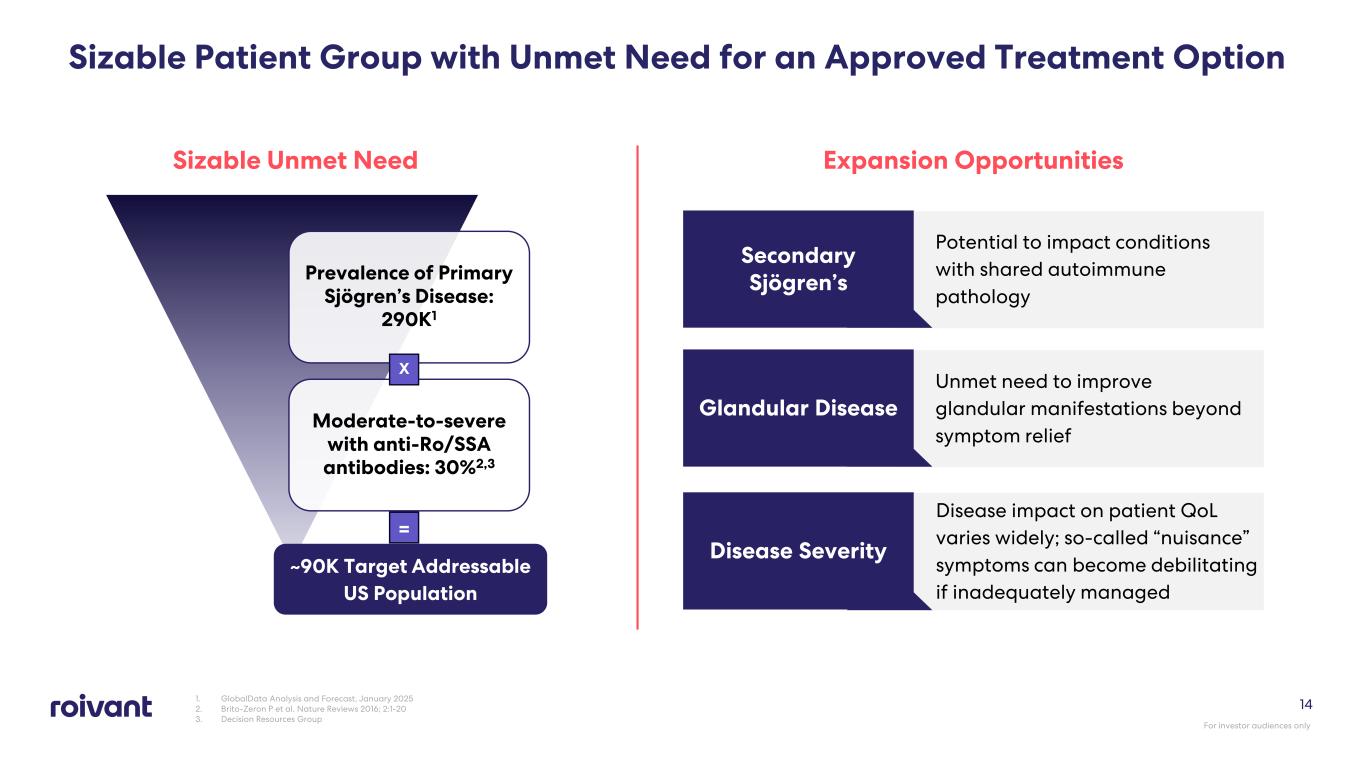
Sizable Patient Group with Unmet Need for an Approved Treatment Option 1. GlobalData Analysis and Forecast, January 2025 2. Brito-Zeron P et al. Nature Reviews 2016; 2:1-20 3. Decision Resources Group Sizable Unmet Need Expansion Opportunities Potential to impact conditions with shared autoimmune pathology Secondary Sjögren’s Unmet need to improve glandular manifestations beyond symptom relief Glandular Disease Disease impact on patient QoL varies widely; so-called “nuisance” symptoms can become debilitating if inadequately managed Disease Severity Prevalence of Primary Sjögren’s Disease: 290K1 Moderate-to-severe with anti-Ro/SSA antibodies: 30%2,3 ~90K Target Addressable US Population X = For investor audiences only 14
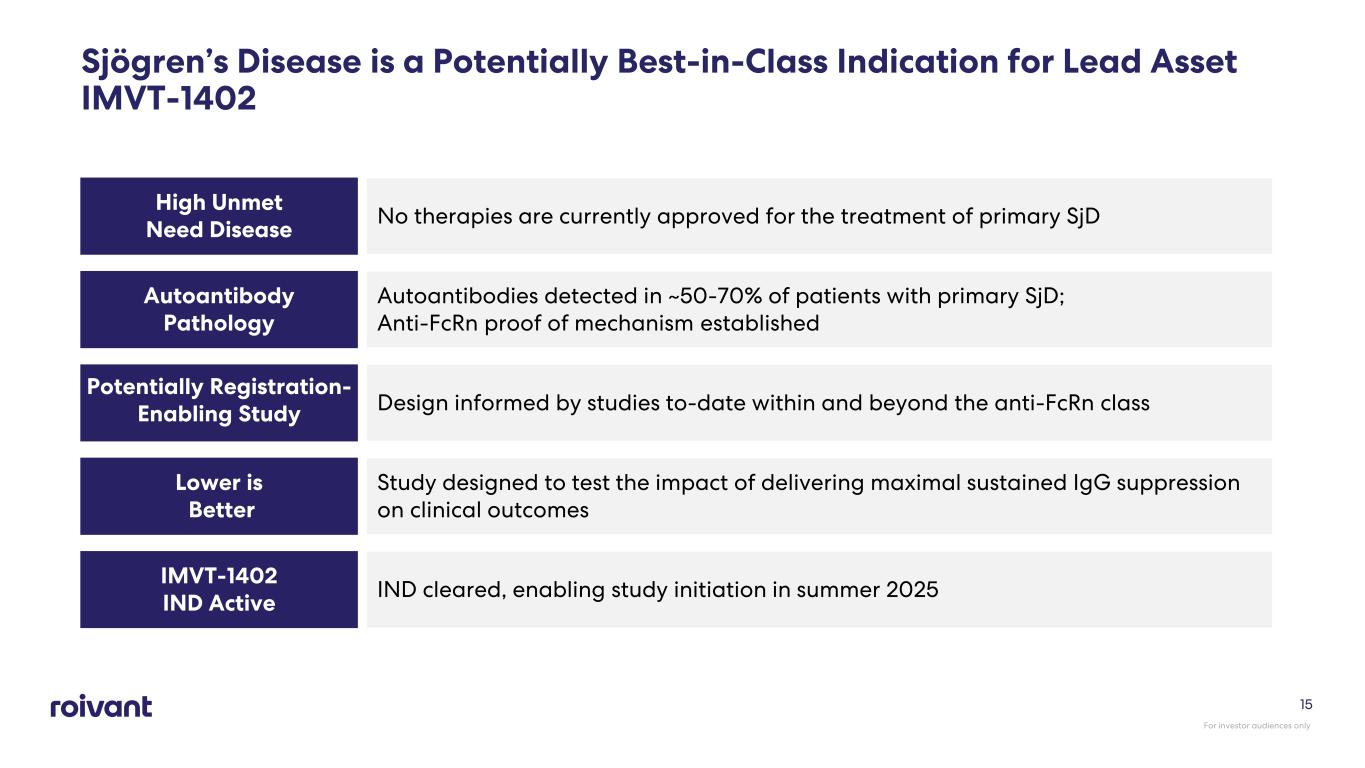
Sjögren’s Disease is a Potentially Best-in-Class Indication for Lead Asset IMVT-1402 High Unmet Need Disease No therapies are currently approved for the treatment of primary SjD Autoantibody Pathology Autoantibodies detected in ~50-70% of patients with primary SjD; Anti-FcRn proof of mechanism established Potentially Registration- Enabling Study Design informed by studies to-date within and beyond the anti-FcRn class Lower is Better Study designed to test the impact of delivering maximal sustained IgG suppression on clinical outcomes IMVT-1402 IND Active IND cleared, enabling study initiation in summer 2025 For investor audiences only 15
Cutaneous Lupus Erythematosus First-/Best-in-Class Opportunity
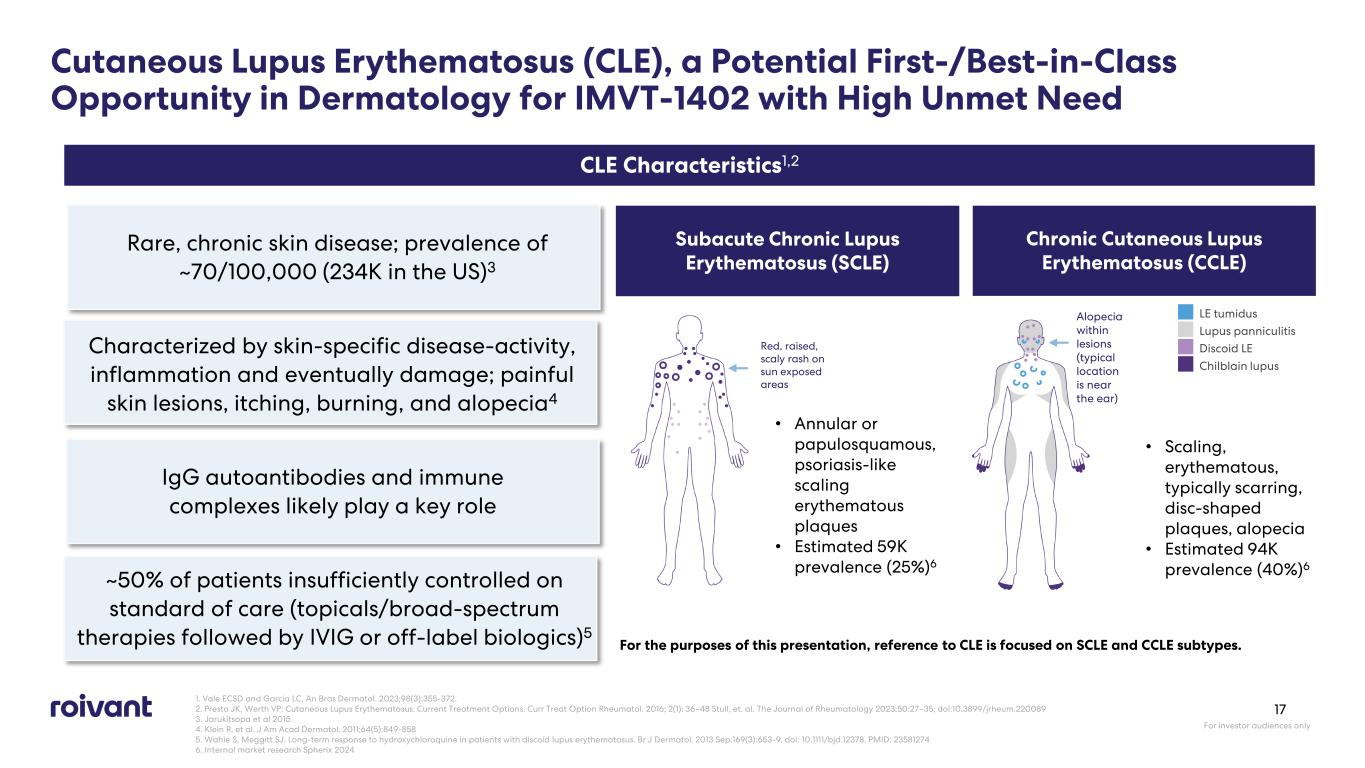
Cutaneous Lupus Erythematosus (CLE), a Potential First-/Best-in-Class Opportunity in Dermatology for IMVT-1402 with High Unmet Need Rare, chronic skin disease; prevalence of ~70/100,000 (234K in the US)3 Characterized by skin-specific disease-activity, inflammation and eventually damage; painful skin lesions, itching, burning, and alopecia4 IgG autoantibodies and immune complexes likely play a key role CLE Characteristics1,2 Subacute Chronic Lupus Erythematosus (SCLE) Chronic Cutaneous Lupus Erythematosus (CCLE) For the purposes of this presentation, reference to CLE is focused on SCLE and CCLE subtypes. • Annular or papulosquamous, psoriasis-like scaling erythematous plaques • Estimated 59K prevalence (25%)6 • Scaling, erythematous, typically scarring, disc-shaped plaques, alopecia • Estimated 94K prevalence (40%)6 LE tumidus Lupus panniculitis Discoid LE Chilblain lupus Alopecia within lesions (typical location is near the ear) Red, raised, scaly rash on sun exposed areas 1. Vale ECSD and Garcia LC. An Bras Dermatol. 2023;98(3):355-372. 2. Presto JK, Werth VP: Cutaneous Lupus Erythematosus: Current Treatment Options. Curr Treat Option Rheumatol. 2016; 2(1): 36–48 Stull, et. al. The Journal of Rheumatology 2023;50:27–35; doi:10.3899/jrheum.220089 3. Jarukitsopa et al 2015 4. Klein R, et al. J Am Acad Dermatol. 2011;64(5):849-858 5. Wahie S, Meggitt SJ. Long-term response to hydroxychloroquine in patients with discoid lupus erythematosus. Br J Dermatol. 2013 Sep;169(3):653-9. doi: 10.1111/bjd.12378. PMID: 23581274 6. Internal market research Spherix 2024 For investor audiences only 17 ~50% of patients insufficiently controlled on standard of care (topicals/broad-spectrum therapies followed by IVIG or off-label biologics)5
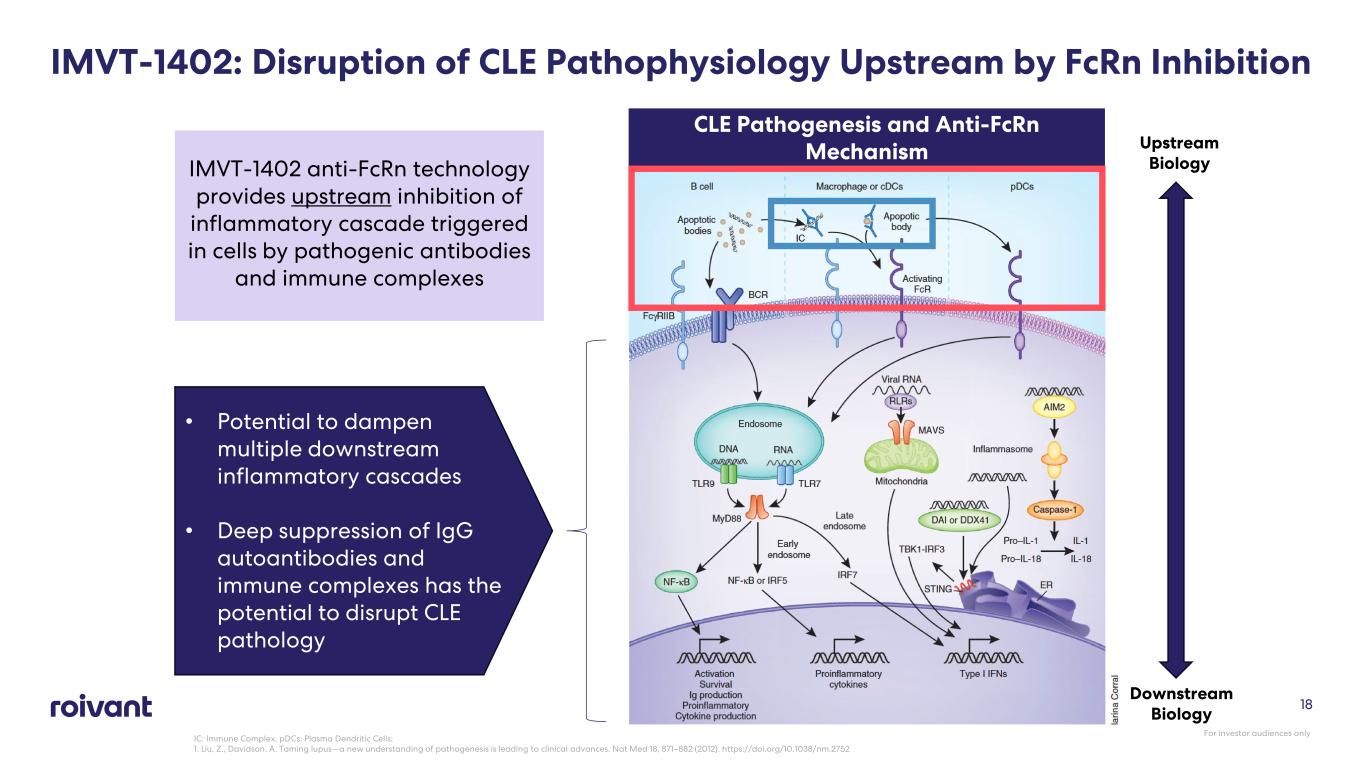
18 IMVT-1402: Disruption of CLE Pathophysiology Upstream by FcRn Inhibition Downstream Biology IMVT-1402 anti-FcRn technology provides upstream inhibition of inflammatory cascade triggered in cells by pathogenic antibodies and immune complexes Upstream Biology • Potential to dampen multiple downstream inflammatory cascades • Deep suppression of IgG autoantibodies and immune complexes has the potential to disrupt CLE pathology CLE Pathogenesis and Anti-FcRn Mechanism IC: Immune Complex, pDCs: Plasma Dendritic Cells; 1. Liu, Z., Davidson, A. Taming lupus—a new understanding of pathogenesis is leading to clinical advances. Nat Med 18, 871–882 (2012). https://doi.org/10.1038/nm.2752 For investor audiences only
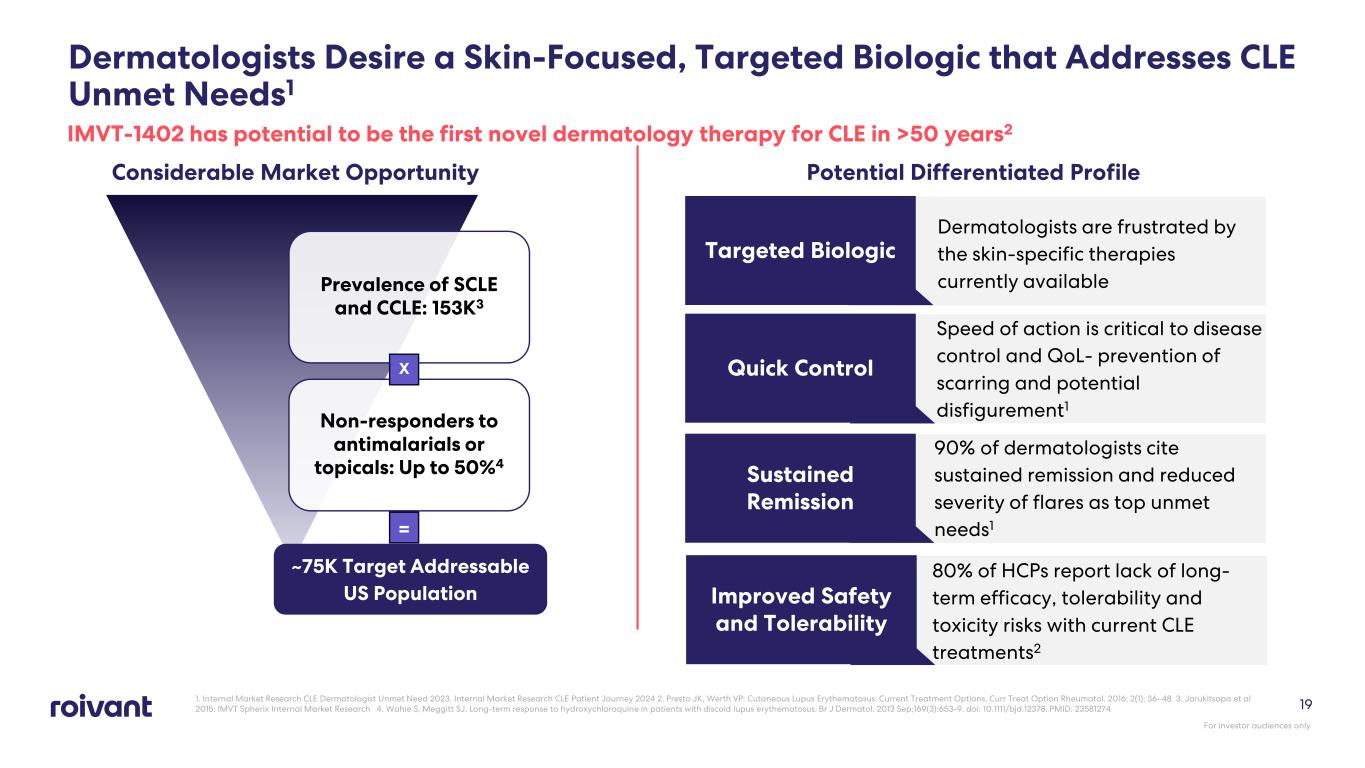
Dermatologists Desire a Skin-Focused, Targeted Biologic that Addresses CLE Unmet Needs1 1. Internal Market Research CLE Dermatologist Unmet Need 2023, Internal Market Research CLE Patient Journey 2024 2. Presto JK, Werth VP: Cutaneous Lupus Erythematosus: Current Treatment Options. Curr Treat Option Rheumatol. 2016; 2(1): 36–48 3. Jarukitsopa et al 2015; IMVT Spherix Internal Market Research 4. Wahie S, Meggitt SJ. Long-term response to hydroxychloroquine in patients with discoid lupus erythematosus. Br J Dermatol. 2013 Sep;169(3):653-9. doi: 10.1111/bjd.12378. PMID: 23581274 Considerable Market Opportunity Potential Differentiated Profile Dermatologists are frustrated by the skin-specific therapies currently available Targeted Biologic Speed of action is critical to disease control and QoL- prevention of scarring and potential disfigurement1 Quick Control 90% of dermatologists cite sustained remission and reduced severity of flares as top unmet needs1 Sustained Remission Prevalence of SCLE and CCLE: 153K3 Non-responders to antimalarials or topicals: Up to 50%4 ~75K Target Addressable US Population X = For investor audiences only 19 IMVT-1402 has potential to be the first novel dermatology therapy for CLE in >50 years2 80% of HCPs report lack of long- term efficacy, tolerability and toxicity risks with current CLE treatments2 Improved Safety and Tolerability
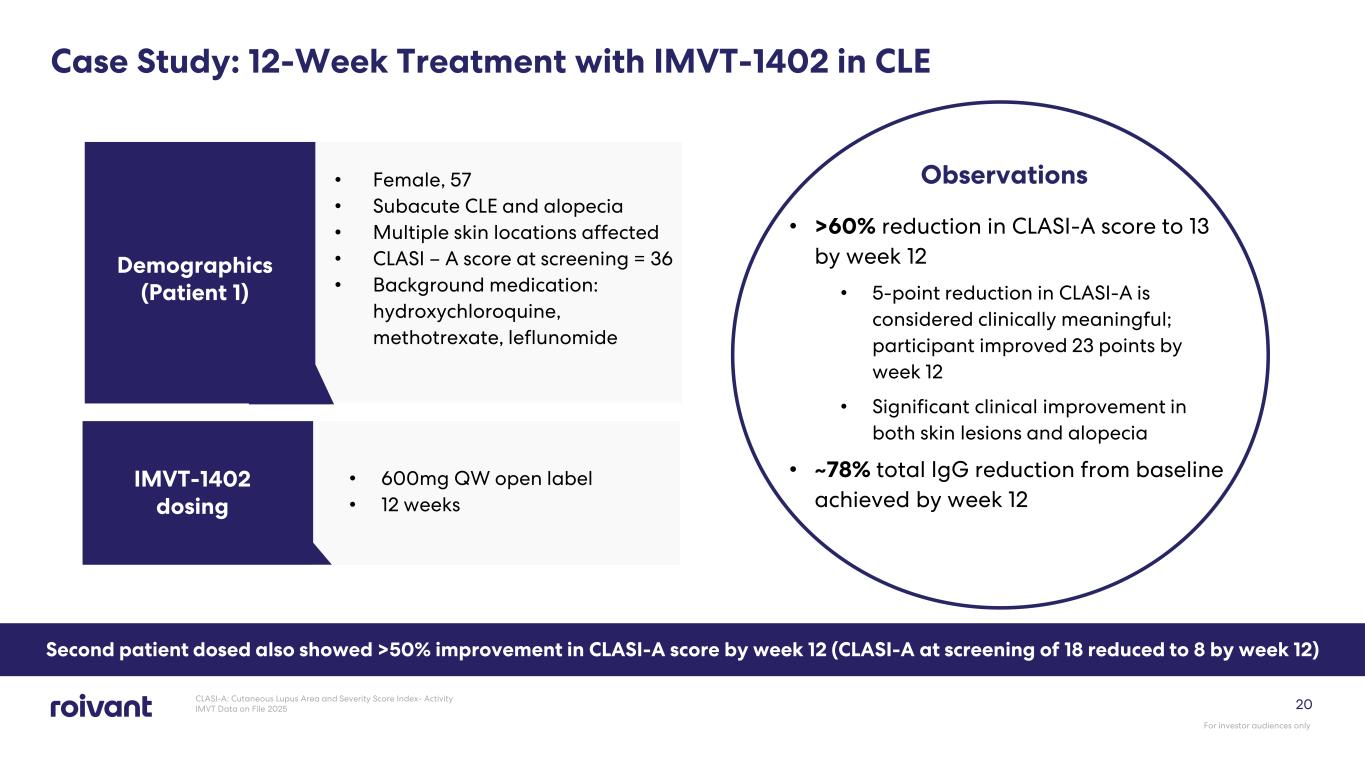
20 Case Study: 12-Week Treatment with IMVT-1402 in CLE • Female, 57 • Subacute CLE and alopecia • Multiple skin locations affected • CLASI – A score at screening = 36 • Background medication: hydroxychloroquine, methotrexate, leflunomide Demographics (Patient 1) • 600mg QW open label • 12 weeks IMVT-1402 dosing Observations • >60% reduction in CLASI-A score to 13 by week 12 • 5-point reduction in CLASI-A is considered clinically meaningful; participant improved 23 points by week 12 • Significant clinical improvement in both skin lesions and alopecia • ~78% total IgG reduction from baseline achieved by week 12 CLASI-A: Cutaneous Lupus Area and Severity Score Index- Activity IMVT Data on File 2025 For investor audiences only Second patient dosed also showed >50% improvement in CLASI-A score by week 12 (CLASI-A at screening of 18 reduced to 8 by week 12)
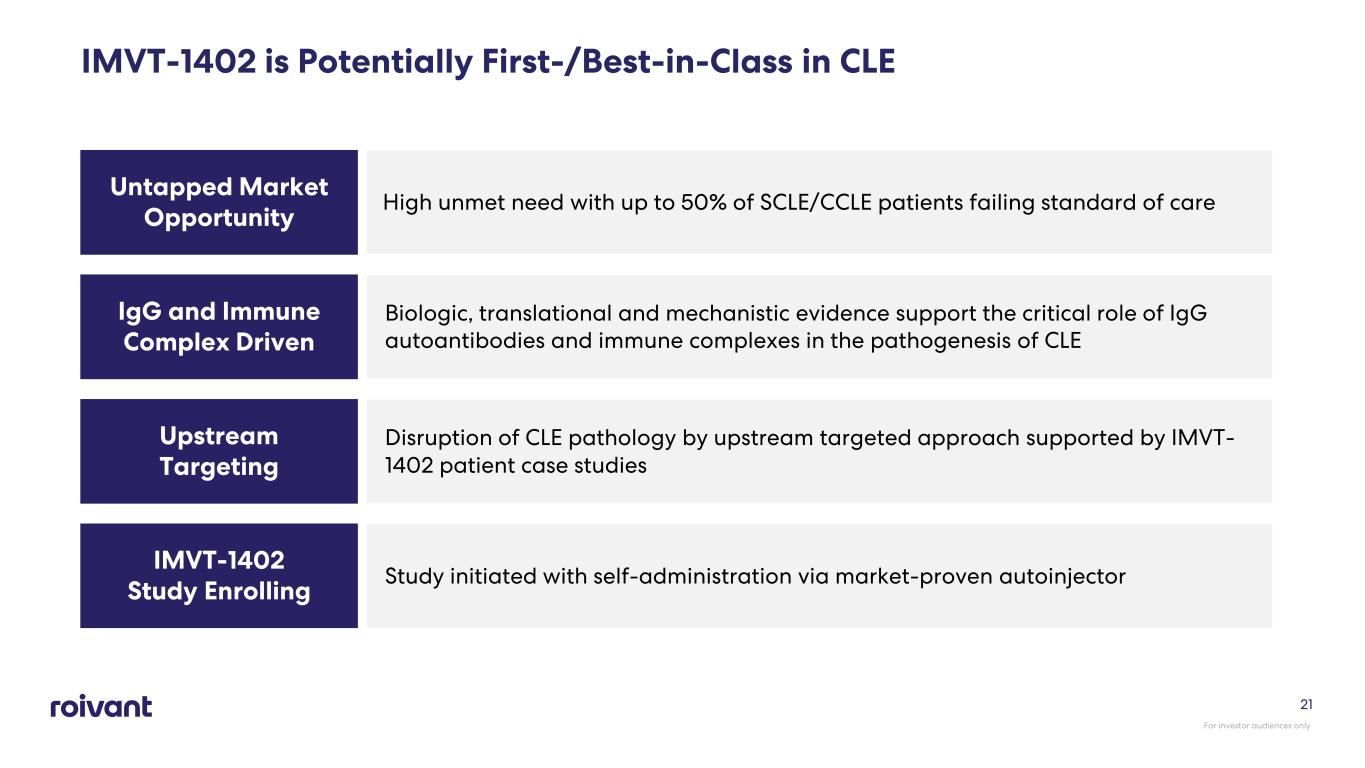
IMVT-1402 is Potentially First-/Best-in-Class in CLE Untapped Market Opportunity High unmet need with up to 50% of SCLE/CCLE patients failing standard of care IgG and Immune Complex Driven Biologic, translational and mechanistic evidence support the critical role of IgG autoantibodies and immune complexes in the pathogenesis of CLE Upstream Targeting Disruption of CLE pathology by upstream targeted approach supported by IMVT- 1402 patient case studies IMVT-1402 Study Enrolling Study initiated with self-administration via market-proven autoinjector For investor audiences only 21
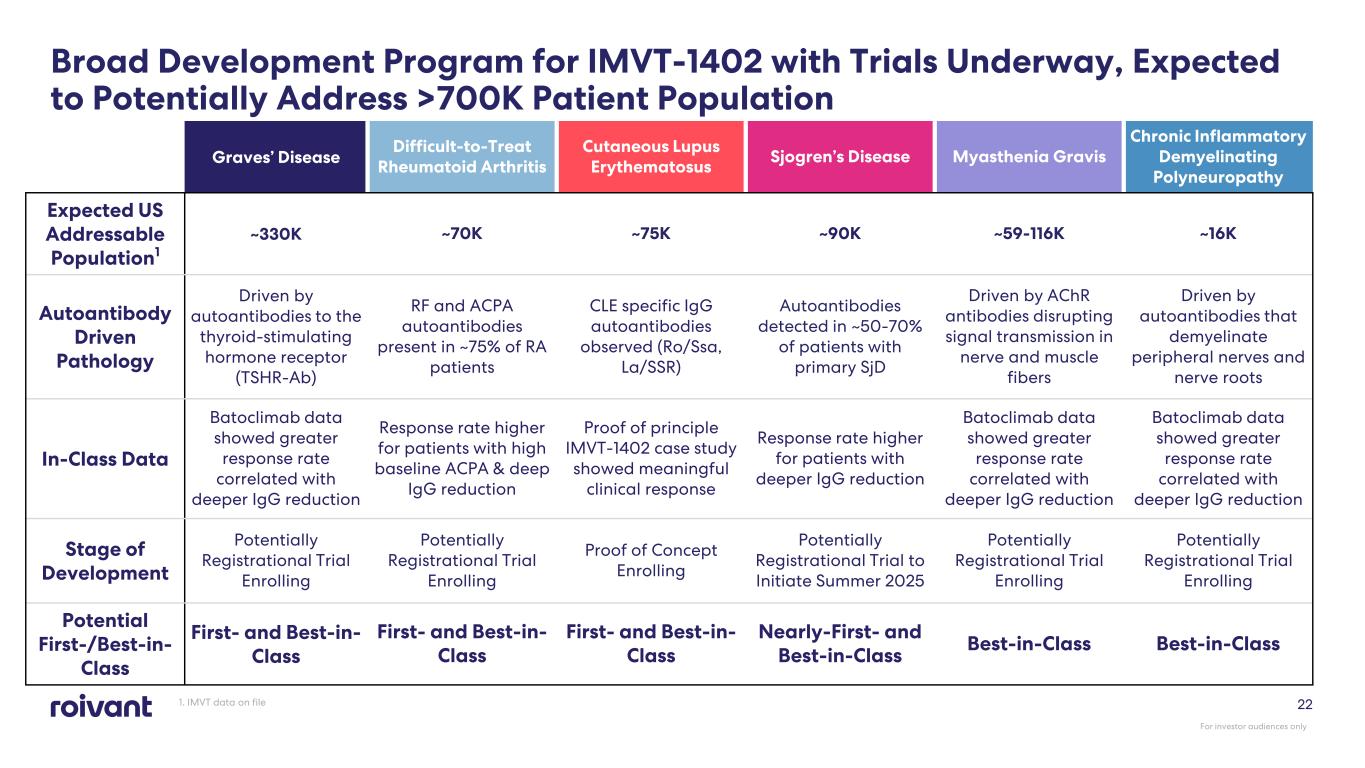
Graves’ Disease Difficult-to-Treat Rheumatoid Arthritis Cutaneous Lupus Erythematosus Sjogren’s Disease Myasthenia Gravis Chronic Inflammatory Demyelinating Polyneuropathy Expected US Addressable Population1 ~330K ~70K ~75K ~90K ~59-116K ~16K Autoantibody Driven Pathology Driven by autoantibodies to the thyroid-stimulating hormone receptor (TSHR-Ab) RF and ACPA autoantibodies present in ~75% of RA patients CLE specific IgG autoantibodies observed (Ro/Ssa, La/SSR) Autoantibodies detected in ~50-70% of patients with primary SjD Driven by AChR antibodies disrupting signal transmission in nerve and muscle fibers Driven by autoantibodies that demyelinate peripheral nerves and nerve roots In-Class Data Batoclimab data showed greater response rate correlated with deeper IgG reduction Response rate higher for patients with high baseline ACPA & deep IgG reduction Proof of principle IMVT-1402 case study showed meaningful clinical response Response rate higher for patients with deeper IgG reduction Batoclimab data showed greater response rate correlated with deeper IgG reduction Batoclimab data showed greater response rate correlated with deeper IgG reduction Stage of Development Potentially Registrational Trial Enrolling Potentially Registrational Trial Enrolling Proof of Concept Enrolling Potentially Registrational Trial to Initiate Summer 2025 Potentially Registrational Trial Enrolling Potentially Registrational Trial Enrolling Potential First-/Best-in- Class First- and Best-in- Class First- and Best-in- Class First- and Best-in- Class Nearly-First- and Best-in-Class Best-in-Class Best-in-Class 22 Broad Development Program for IMVT-1402 with Trials Underway, Expected to Potentially Address >700K Patient Population 1. IMVT data on file For investor audiences only
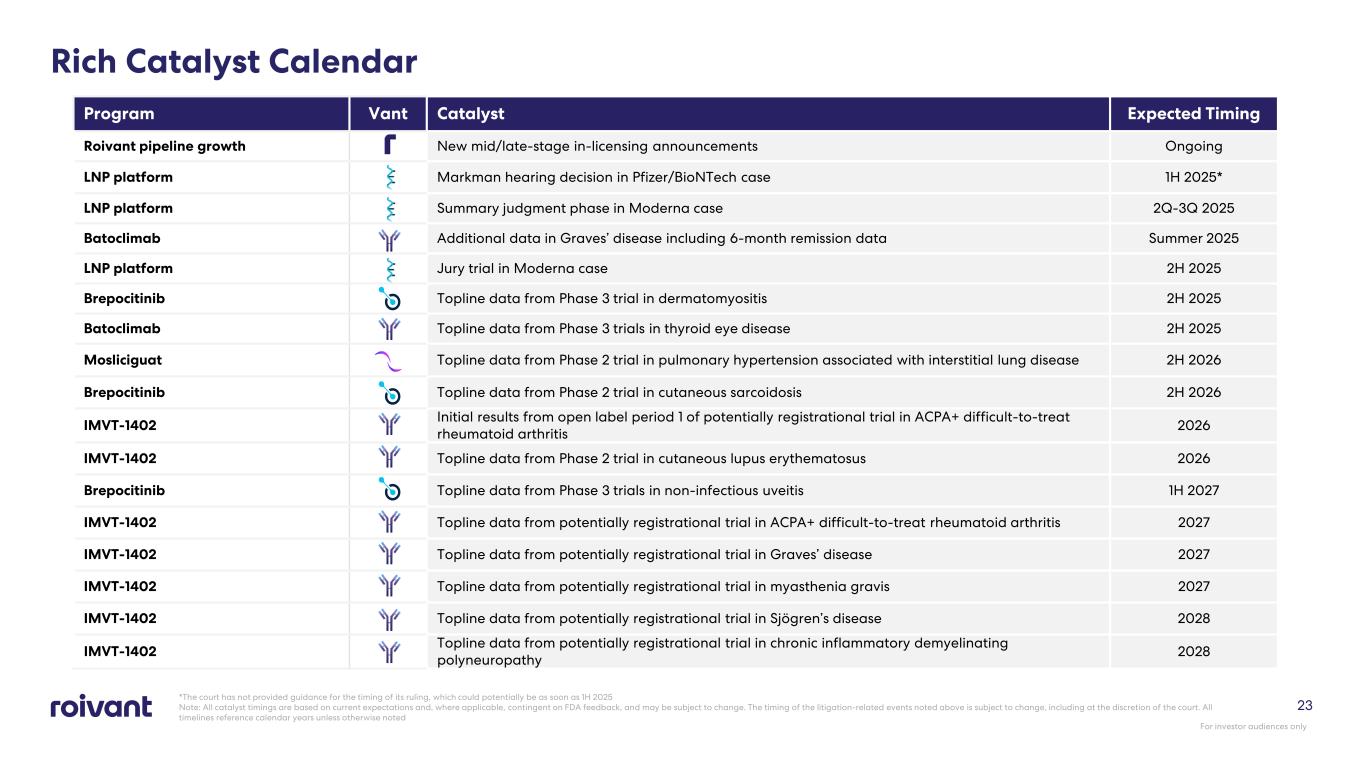
23 Rich Catalyst Calendar *The court has not provided guidance for the timing of its ruling, which could potentially be as soon as 1H 2025 Note: All catalyst timings are based on current expectations and, where applicable, contingent on FDA feedback, and may be subject to change. The timing of the litigation-related events noted above is subject to change, including at the discretion of the court. All timelines reference calendar years unless otherwise noted Program Vant Catalyst Expected Timing Roivant pipeline growth New mid/late-stage in-licensing announcements Ongoing LNP platform Markman hearing decision in Pfizer/BioNTech case 1H 2025* LNP platform Summary judgment phase in Moderna case 2Q-3Q 2025 Batoclimab Additional data in Graves’ disease including 6-month remission data Summer 2025 LNP platform Jury trial in Moderna case 2H 2025 Brepocitinib Topline data from Phase 3 trial in dermatomyositis 2H 2025 Batoclimab Topline data from Phase 3 trials in thyroid eye disease 2H 2025 Mosliciguat Topline data from Phase 2 trial in pulmonary hypertension associated with interstitial lung disease 2H 2026 Brepocitinib Topline data from Phase 2 trial in cutaneous sarcoidosis 2H 2026 IMVT-1402 Initial results from open label period 1 of potentially registrational trial in ACPA+ difficult-to-treat rheumatoid arthritis 2026 IMVT-1402 Topline data from Phase 2 trial in cutaneous lupus erythematosus 2026 Brepocitinib Topline data from Phase 3 trials in non-infectious uveitis 1H 2027 IMVT-1402 Topline data from potentially registrational trial in ACPA+ difficult-to-treat rheumatoid arthritis 2027 IMVT-1402 Topline data from potentially registrational trial in Graves’ disease 2027 IMVT-1402 Topline data from potentially registrational trial in myasthenia gravis 2027 IMVT-1402 Topline data from potentially registrational trial in Sjögren’s disease 2028 IMVT-1402 Topline data from potentially registrational trial in chronic inflammatory demyelinating polyneuropathy 2028 For investor audiences only

Thank you. 24

Appendix 25
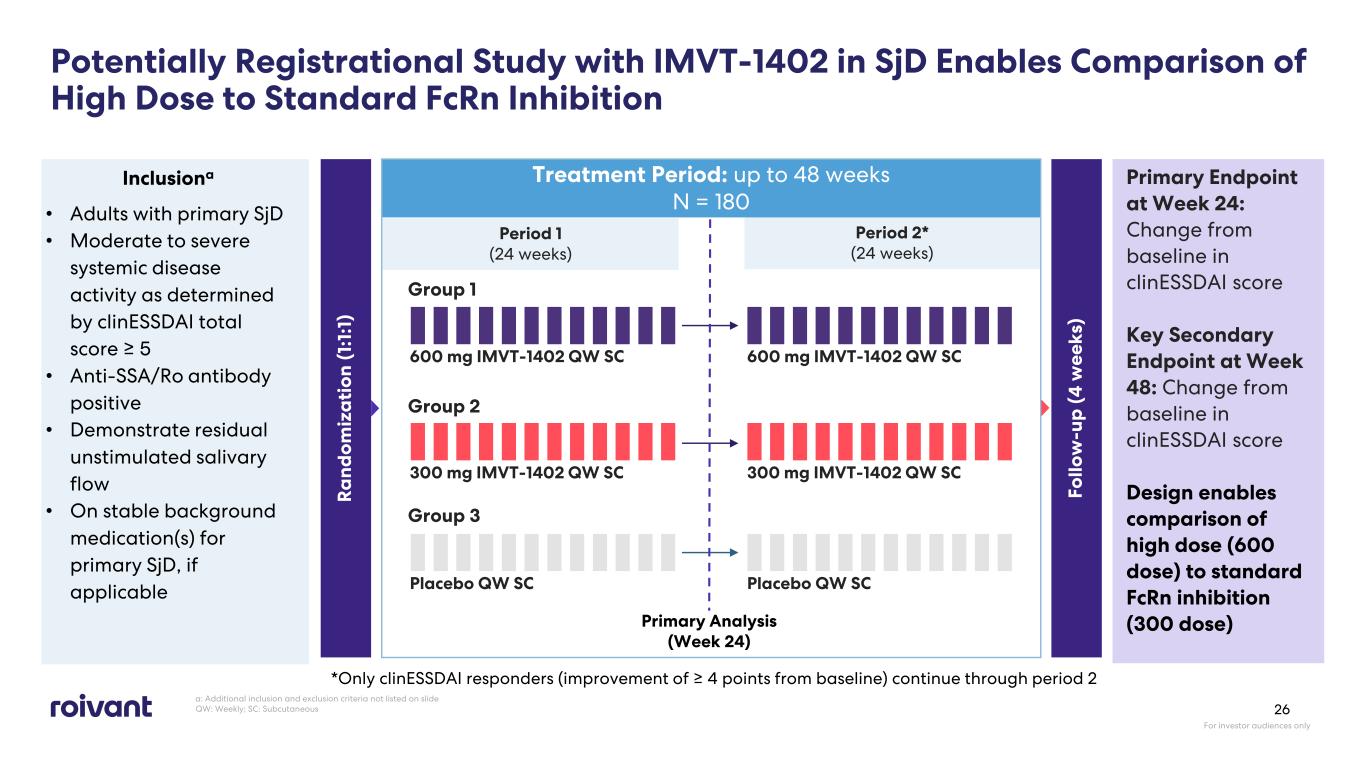
Potentially Registrational Study with IMVT-1402 in SjD Enables Comparison of High Dose to Standard FcRn Inhibition Treatment Period: up to 48 weeks N = 180 Primary Endpoint at Week 24: Change from baseline in clinESSDAI score Key Secondary Endpoint at Week 48: Change from baseline in clinESSDAI score Design enables comparison of high dose (600 dose) to standard FcRn inhibition (300 dose) Inclusiona Group 1 Group 3 Group 2 Period 2* (24 weeks) Period 1 (24 weeks) 300 mg IMVT-1402 QW SC 600 mg IMVT-1402 QW SC 600 mg IMVT-1402 QW SC Placebo QW SC Placebo QW SC Fo llo w -u p (4 w ee ks ) 300 mg IMVT-1402 QW SC *Only clinESSDAI responders (improvement of ≥ 4 points from baseline) continue through period 2 R a nd om iz a ti on ( 1: 1: 1) • Adults with primary SjD • Moderate to severe systemic disease activity as determined by clinESSDAI total score ≥ 5 • Anti-SSA/Ro antibody positive • Demonstrate residual unstimulated salivary flow • On stable background medication(s) for primary SjD, if applicable a: Additional inclusion and exclusion criteria not listed on slide QW: Weekly; SC: Subcutaneous For investor audiences only 26 Primary Analysis (Week 24)
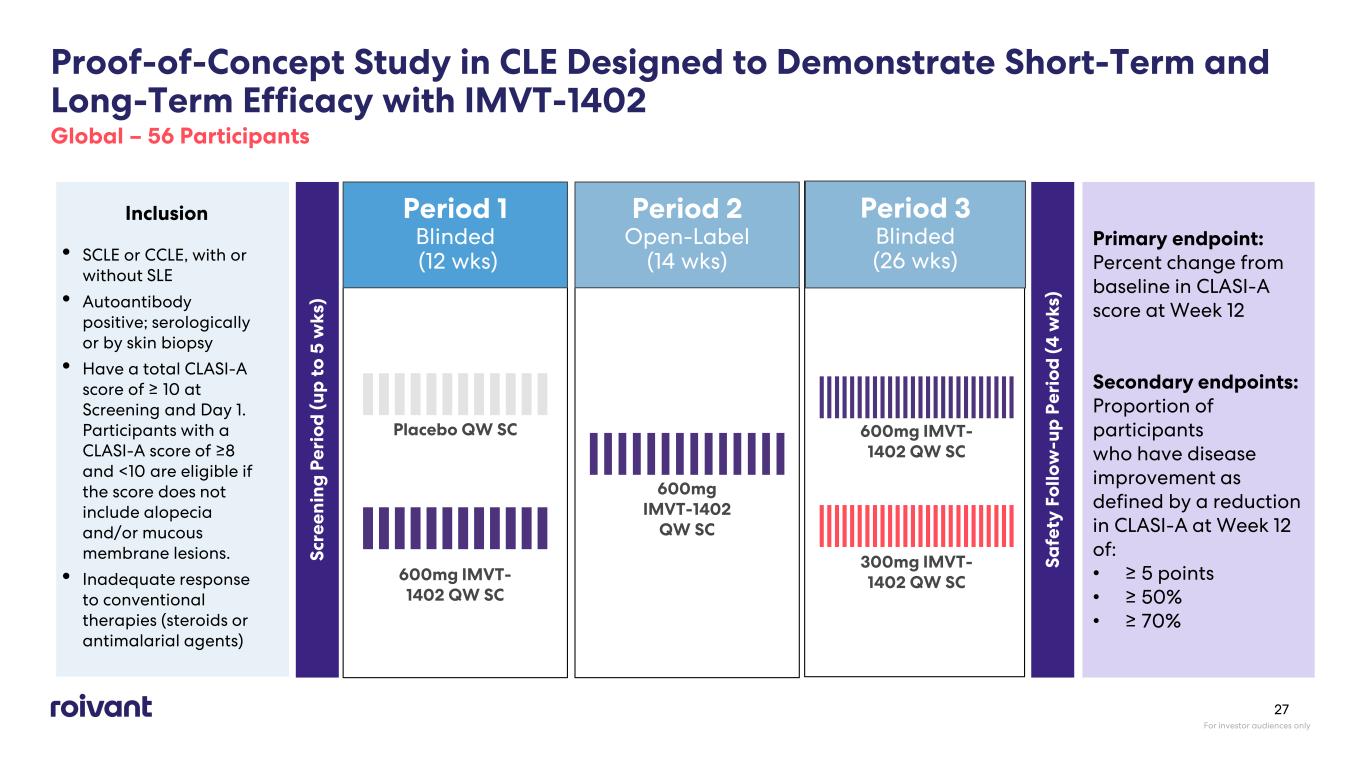
Period 2 Open-Label (14 wks) Period 1 Blinded (12 wks) Proof-of-Concept Study in CLE Designed to Demonstrate Short-Term and Long-Term Efficacy with IMVT-1402 Global – 56 Participants Inclusion • SCLE or CCLE, with or without SLE • Autoantibody positive; serologically or by skin biopsy • Have a total CLASI-A score of ≥ 10 at Screening and Day 1. Participants with a CLASI-A score of ≥8 and <10 are eligible if the score does not include alopecia and/or mucous membrane lesions. • Inadequate response to conventional therapies (steroids or antimalarial agents) Sc re en in g P er io d (u p t o 5 w ks ) 600mg IMVT- 1402 QW SC Primary endpoint: Percent change from baseline in CLASI-A score at Week 12 Secondary endpoints: Proportion of participants who have disease improvement as defined by a reduction in CLASI-A at Week 12 of: • ≥ 5 points • ≥ 50% • ≥ 70% Sa fe ty F ol lo w -u p P er io d (4 w ks ) Placebo QW SC 600mg IMVT-1402 QW SC Period 3 Blinded (26 wks) 600mg IMVT- 1402 QW SC 300mg IMVT- 1402 QW SC For investor audiences only 27