
Targeted science, Tailored solutions for people with autoimmune disease MG & CIDP Results March 2025 Exhibit 99.2

Forward-looking statements This presentation contains forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. The use of words such as "can," “may,” “might,” “will,” “would,” “should,” “expect,” “believe,” “estimate,” “design,” “plan,” "intend," "anticipate," and other similar expressions are intended to identify forward-looking statements. Such forward looking statements include Immunovant’s expectations regarding the goals of its clinical development programs, including the efficacy, safety, and clinical success of batoclimab in Immunovant’s myasthenia gravis (MG) and chronic inflammatory demyelinating polyneuropathy (CIDP) programs; belief in the performance, magnitude of benefit, or best-in-class results shown with batoclimab relative to therapies evaluated in other trials; plans and expectations for a pivotal trial of IMVT-1402 in MG, including the timing thereof; expectations regarding the potential for IMVT-1402 to meet or exceed the results observed in studies of batoclimab; beliefs regarding the best-in-class potential of IMVT-1402; and the anticipated benefits of Immunovant’s strategic reprioritization from batoclimab to IMVT-1402. All forward-looking statements are based on estimates and assumptions by Immunovant’s management that, although Immunovant believes to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that Immunovant expected. Such risks and uncertainties include, among others: initial results or other preliminary analyses or results of early clinical trials may not be predictive of final trial results or of the results of later clinical trials; results of animal studies may not be predictive of results in humans; the timing and availability of data from clinical trials; the timing of discussions with regulatory agencies, as well as regulatory submissions and potential approvals; the continued development of Immunovant’s product candidates, including the timing of the commencement of additional clinical trials; Immunovant’s scientific approach, clinical trial design, indication selection, and general development progress; future clinical trials may not confirm any safety, potency, or other product characteristics described or assumed in this presentation; any product candidate that Immunovant develops may not progress through clinical development or receive required regulatory approvals within expected timelines or at all; Immunovant’s product candidates may not be beneficial to patients, or even if approved by regulatory authorities, successfully commercialized; the effect of global factors such as geopolitical tensions and adverse macroeconomic conditions on Immunovant’s business operations and supply chains, including its clinical development plans and timelines; Immunovant’s business is heavily dependent on the successful development, regulatory approval and commercialization of batoclimab and IMVT-1402; Immunovant is in various stages of clinical development for IMVT-1402 and batoclimab; Immunovant’s intellectual property position; and Immunovant will require additional capital to fund its operations and advance IMVT-1402 and batoclimab through clinical development. These and other risks and uncertainties are more fully described in Immunovant’s periodic and other reports filed with the Securities and Exchange Commission (SEC), including in the section titled “Risk Factors” in Immunovant’s most recent Quarterly Report on Form 10-Q for the quarter ended December 31, 2024, filed with the SEC on February 6, 2025, and Immunovant’s subsequent filings with the SEC. Any forward-looking statement speaks only as of the date on which it was made. Immunovant undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products.
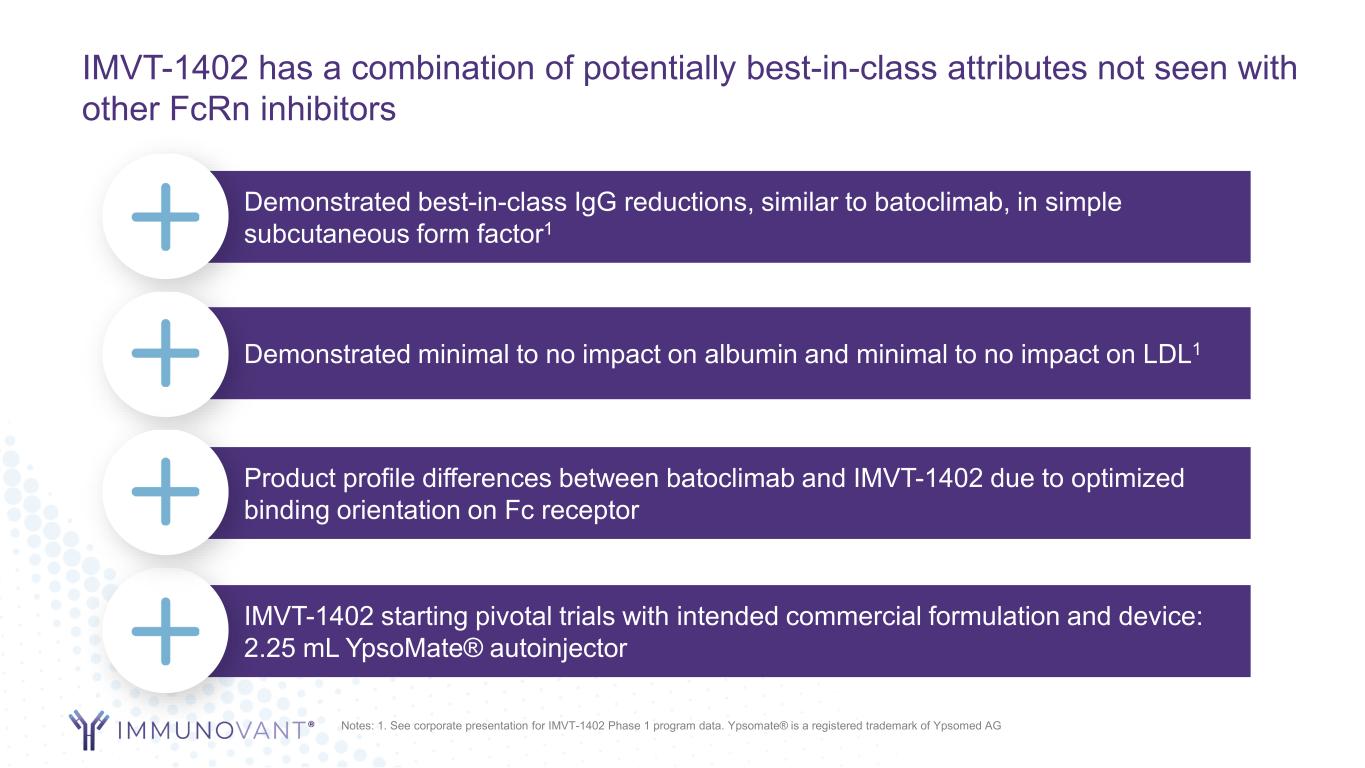
IMVT-1402 has a combination of potentially best-in-class attributes not seen with other FcRn inhibitors Demonstrated best-in-class IgG reductions, similar to batoclimab, in simple subcutaneous form factor1 Demonstrated minimal to no impact on albumin and minimal to no impact on LDL1 IMVT-1402 starting pivotal trials with intended commercial formulation and device: 2.25 mL YpsoMate® autoinjector Product profile differences between batoclimab and IMVT-1402 due to optimized binding orientation on Fc receptor Notes: 1. See corporate presentation for IMVT-1402 Phase 1 program data. Ypsomate® is a registered trademark of Ypsomed AG
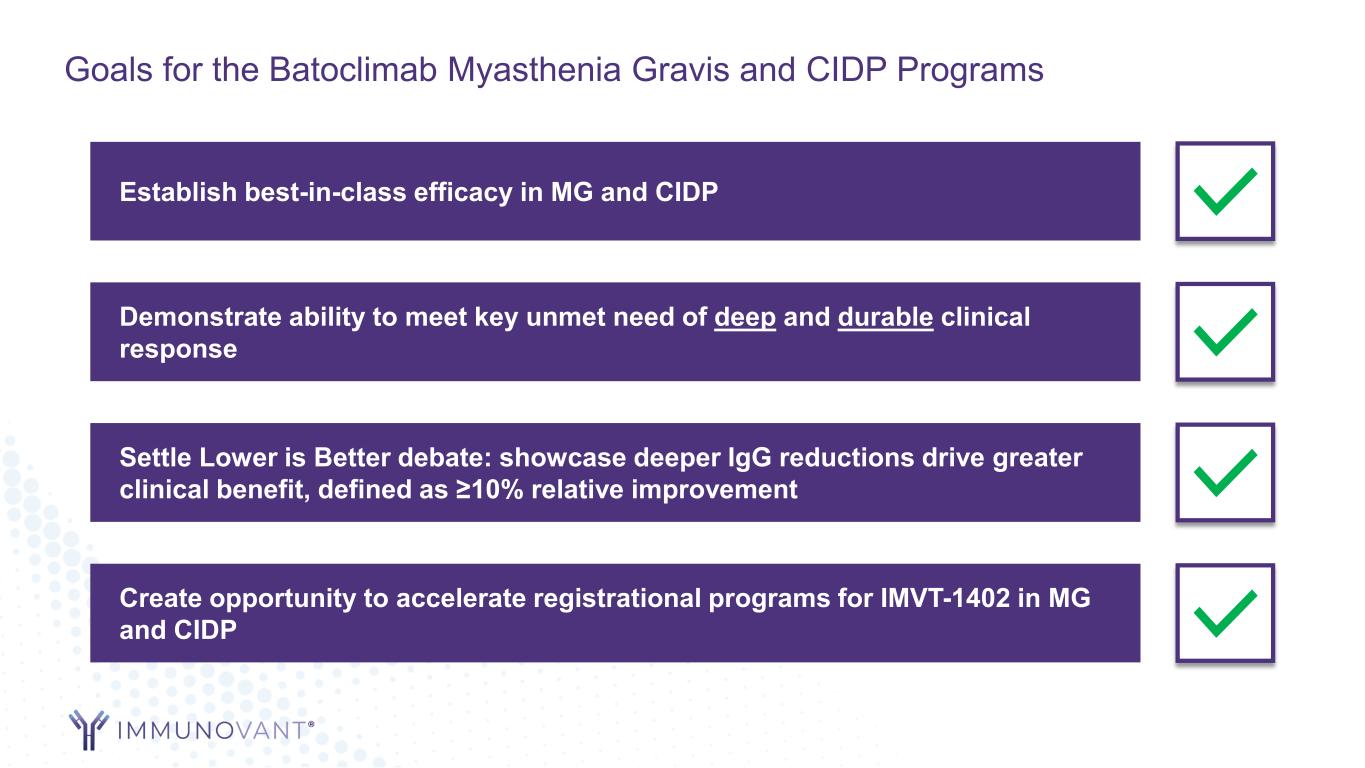
Create opportunity to accelerate registrational programs for IMVT-1402 in MG and CIDP Demonstrate ability to meet key unmet need of deep and durable clinical response Establish best-in-class efficacy in MG and CIDP Goals for the Batoclimab Myasthenia Gravis and CIDP Programs Settle Lower is Better debate: showcase deeper IgG reductions drive greater clinical benefit, defined as ≥10% relative improvement

MG Topline Results
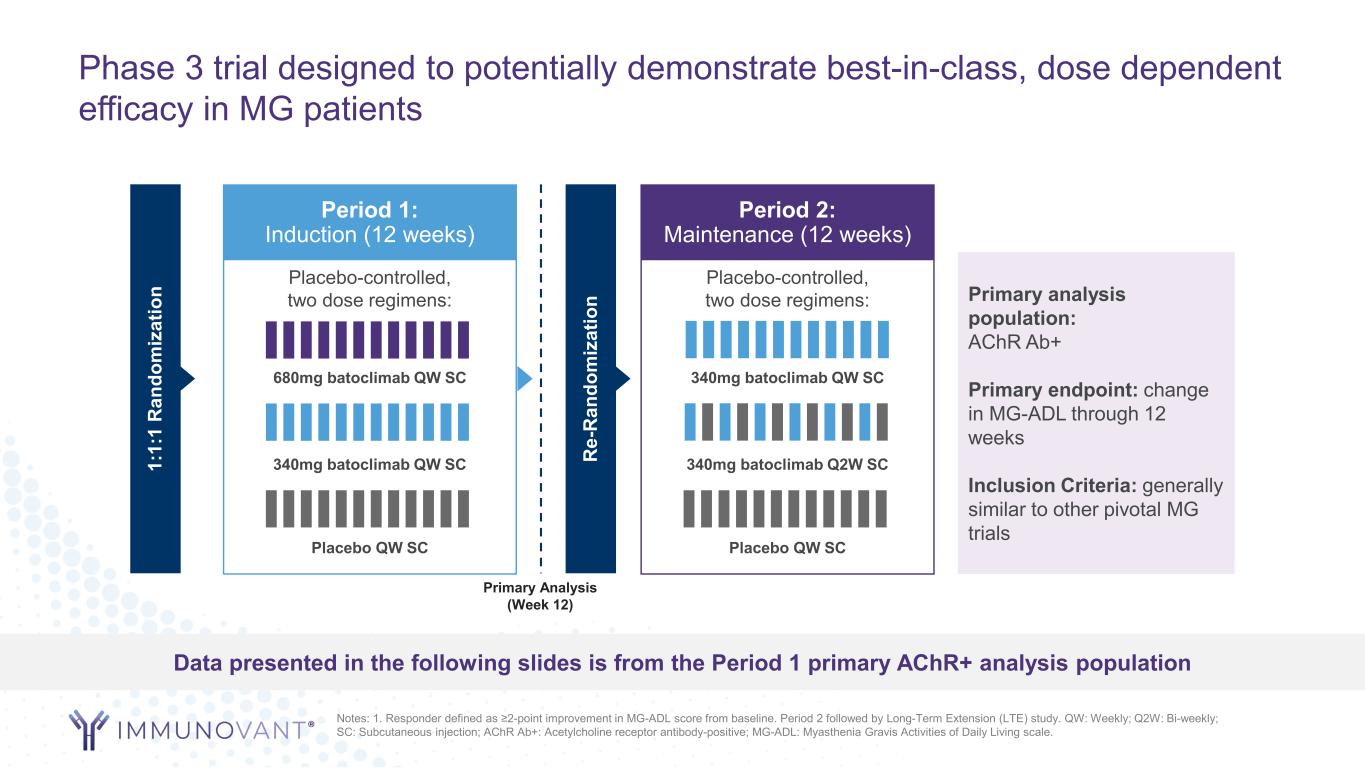
Phase 3 trial designed to potentially demonstrate best-in-class, dose dependent efficacy in MG patients Notes: 1. Responder defined as ≥2-point improvement in MG-ADL score from baseline. Period 2 followed by Long-Term Extension (LTE) study. QW: Weekly; Q2W: Bi-weekly; SC: Subcutaneous injection; AChR Ab+: Acetylcholine receptor antibody-positive; MG-ADL: Myasthenia Gravis Activities of Daily Living scale. Data presented in the following slides is from the Period 1 primary AChR+ analysis population Period 1: Induction (12 weeks) 1: 1: 1 R an do m iz at io n Placebo-controlled, two dose regimens: Placebo QW SC 340mg batoclimab QW SC 680mg batoclimab QW SC Period 2: Maintenance (12 weeks) R e- R an do m iz at io n Placebo-controlled, two dose regimens: Placebo QW SC 340mg batoclimab Q2W SC 340mg batoclimab QW SC Primary analysis population: AChR Ab+ Primary endpoint: change in MG-ADL through 12 weeks Inclusion Criteria: generally similar to other pivotal MG trials Primary Analysis (Week 12)
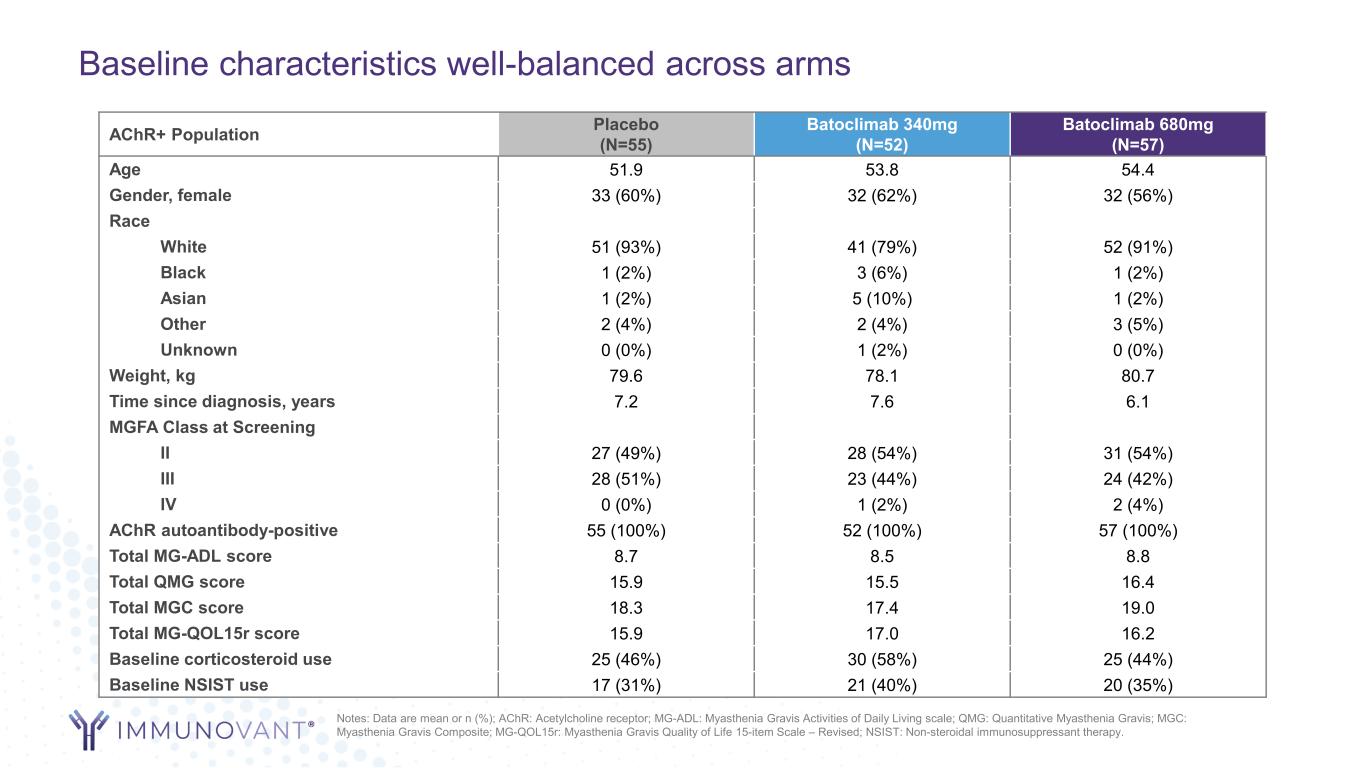
Baseline characteristics well-balanced across arms AChR+ Population Placebo (N=55) Batoclimab 340mg (N=52) Batoclimab 680mg (N=57) Age 51.9 53.8 54.4 Gender, female 33 (60%) 32 (62%) 32 (56%) Race White 51 (93%) 41 (79%) 52 (91%) Black 1 (2%) 3 (6%) 1 (2%) Asian 1 (2%) 5 (10%) 1 (2%) Other 2 (4%) 2 (4%) 3 (5%) Unknown 0 (0%) 1 (2%) 0 (0%) Weight, kg 79.6 78.1 80.7 Time since diagnosis, years 7.2 7.6 6.1 MGFA Class at Screening II 27 (49%) 28 (54%) 31 (54%) III 28 (51%) 23 (44%) 24 (42%) IV 0 (0%) 1 (2%) 2 (4%) AChR autoantibody-positive 55 (100%) 52 (100%) 57 (100%) Total MG-ADL score 8.7 8.5 8.8 Total QMG score 15.9 15.5 16.4 Total MGC score 18.3 17.4 19.0 Total MG-QOL15r score 15.9 17.0 16.2 Baseline corticosteroid use 25 (46%) 30 (58%) 25 (44%) Baseline NSIST use 17 (31%) 21 (40%) 20 (35%) Notes: Data are mean or n (%); AChR: Acetylcholine receptor; MG-ADL: Myasthenia Gravis Activities of Daily Living scale; QMG: Quantitative Myasthenia Gravis; MGC: Myasthenia Gravis Composite; MG-QOL15r: Myasthenia Gravis Quality of Life 15-item Scale – Revised; NSIST: Non-steroidal immunosuppressant therapy.
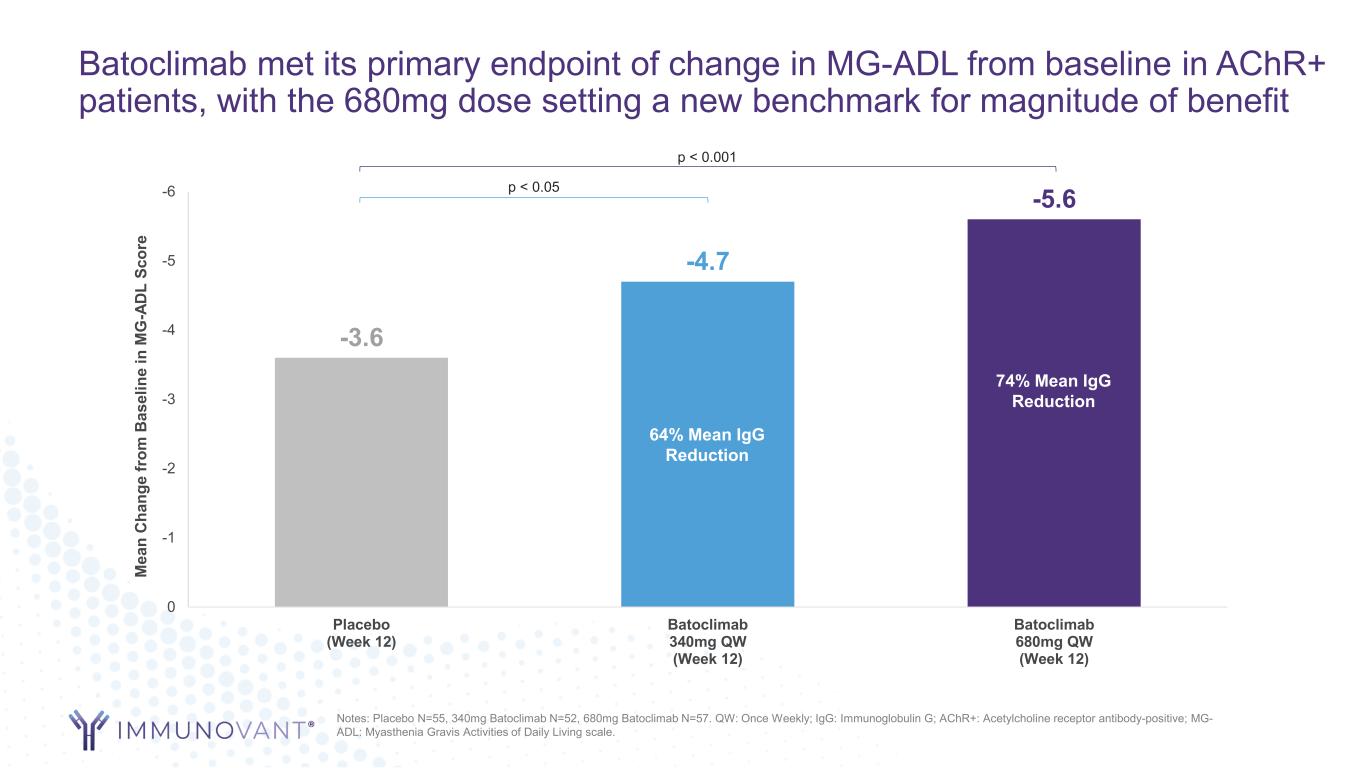
Batoclimab met its primary endpoint of change in MG-ADL from baseline in AChR+ patients, with the 680mg dose setting a new benchmark for magnitude of benefit -3.6 -4.7 -5.6-6 -5 -4 -3 -2 -1 0 Placebo (Week 12) Batoclimab 340mg QW (Week 12) Batoclimab 680mg QW (Week 12) M ea n C ha ng e fr om B as el in e in M G -A D L Sc or e 74% Mean IgG Reduction 64% Mean IgG Reduction p < 0.001 p < 0.05 Notes: Placebo N=55, 340mg Batoclimab N=52, 680mg Batoclimab N=57. QW: Once Weekly; IgG: Immunoglobulin G; AChR+: Acetylcholine receptor antibody-positive; MG- ADL: Myasthenia Gravis Activities of Daily Living scale.
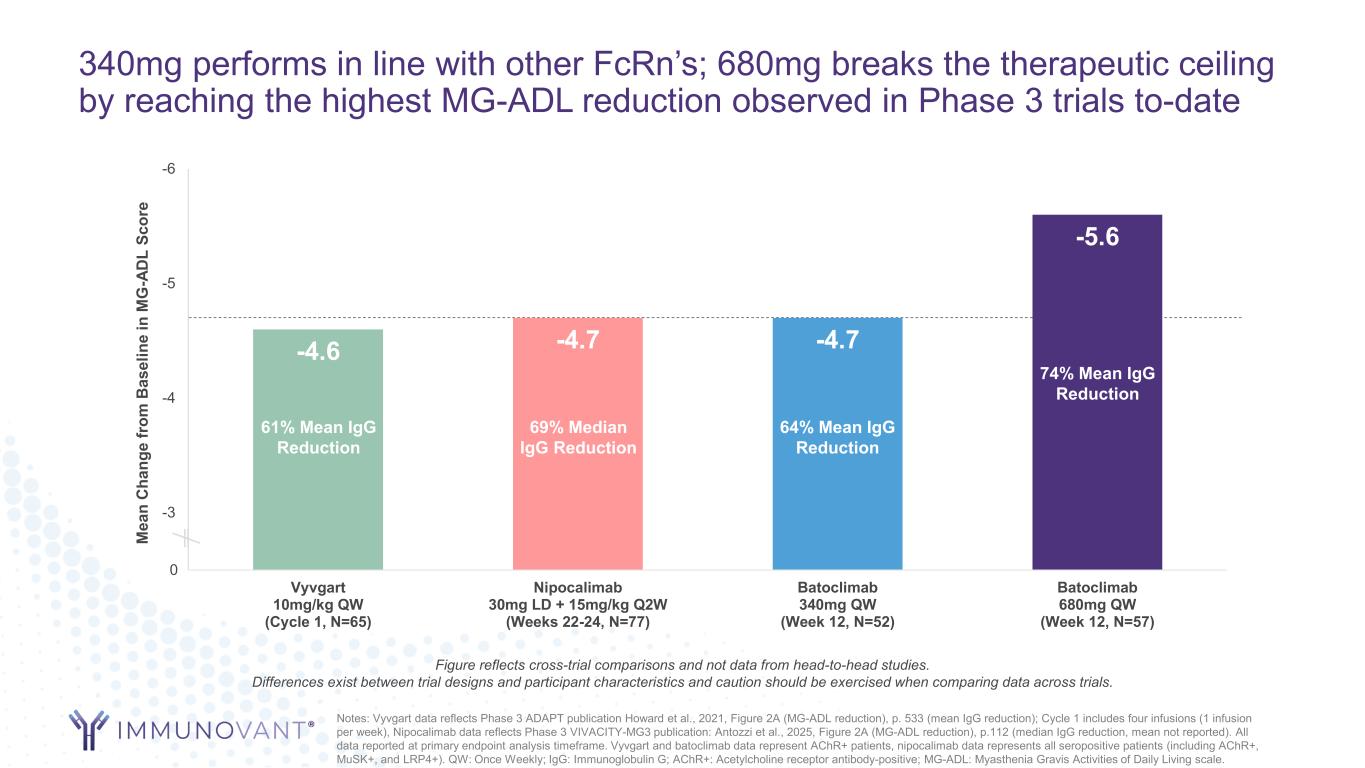
340mg performs in line with other FcRn’s; 680mg breaks the therapeutic ceiling by reaching the highest MG-ADL reduction observed in Phase 3 trials to-date Notes: Vyvgart data reflects Phase 3 ADAPT publication Howard et al., 2021, Figure 2A (MG-ADL reduction), p. 533 (mean IgG reduction); Cycle 1 includes four infusions (1 infusion per week), Nipocalimab data reflects Phase 3 VIVACITY-MG3 publication: Antozzi et al., 2025, Figure 2A (MG-ADL reduction), p.112 (median IgG reduction, mean not reported). All data reported at primary endpoint analysis timeframe. Vyvgart and batoclimab data represent AChR+ patients, nipocalimab data represents all seropositive patients (including AChR+, MuSK+, and LRP4+). QW: Once Weekly; IgG: Immunoglobulin G; AChR+: Acetylcholine receptor antibody-positive; MG-ADL: Myasthenia Gravis Activities of Daily Living scale. -4.6 -4.7 -4.7 -5.6 -6 -5 -4 -3 Vyvgart 10mg/kg QW (Cycle 1, N=65) Nipocalimab 30mg LD + 15mg/kg Q2W (Weeks 22-24, N=77) Batoclimab 340mg QW (Week 12, N=52) Batoclimab 680mg QW (Week 12, N=57) M ea n C ha ng e fr om B as el in e in M G -A D L Sc or e 0 74% Mean IgG Reduction 64% Mean IgG Reduction 69% Median IgG Reduction 61% Mean IgG Reduction Figure reflects cross-trial comparisons and not data from head-to-head studies. Differences exist between trial designs and participant characteristics and caution should be exercised when comparing data across trials.
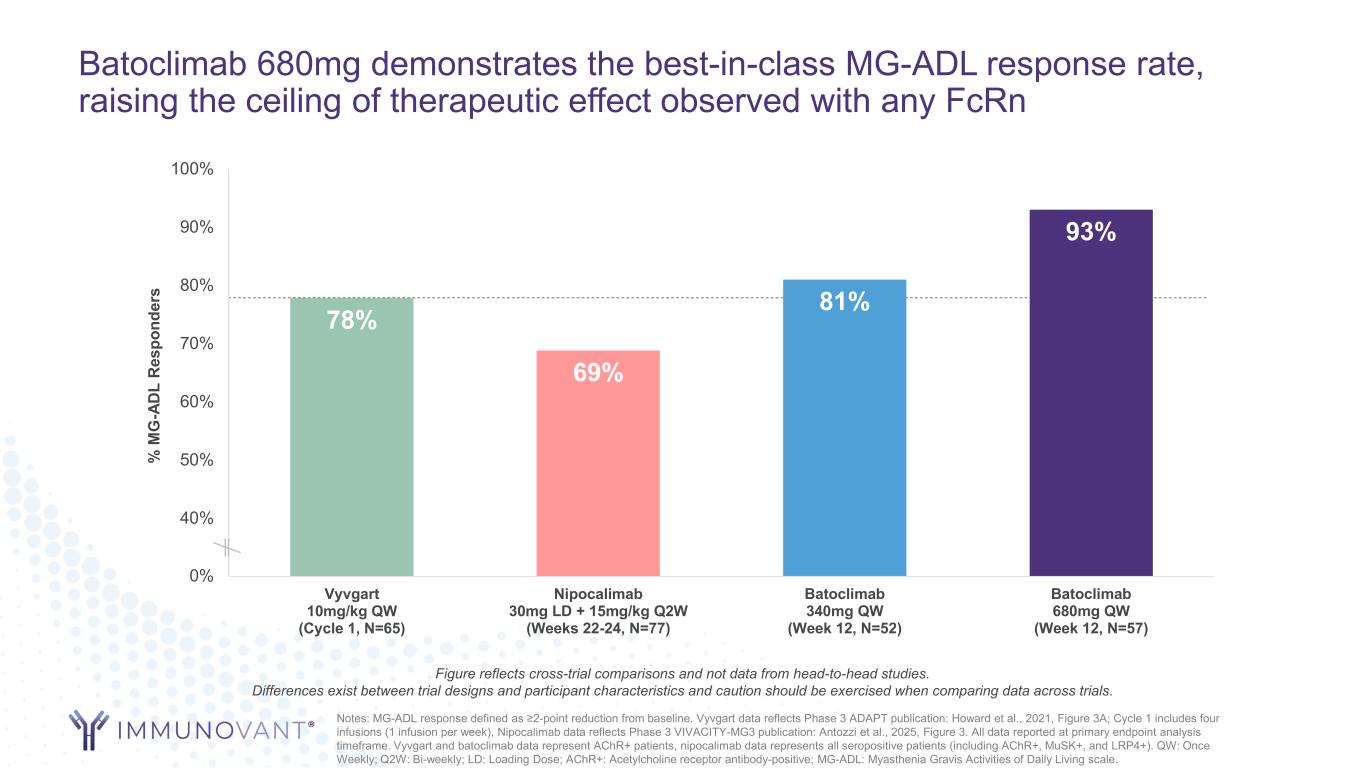
Batoclimab 680mg demonstrates the best-in-class MG-ADL response rate, raising the ceiling of therapeutic effect observed with any FcRn 78% 69% 81% 93% 30% 40% 50% 60% 70% 80% 90% 100% Vyvgart 10mg/kg QW (Cycle 1, N=65) Nipocalimab 30mg LD + 15mg/kg Q2W (Weeks 22-24, N=77) Batoclimab 340mg QW (Week 12, N=52) Batoclimab 680mg QW (Week 12, N=57) % M G -A D L R es po nd er s Notes: MG-ADL response defined as ≥2-point reduction from baseline. Vyvgart data reflects Phase 3 ADAPT publication: Howard et al., 2021, Figure 3A; Cycle 1 includes four infusions (1 infusion per week), Nipocalimab data reflects Phase 3 VIVACITY-MG3 publication: Antozzi et al., 2025, Figure 3. All data reported at primary endpoint analysis timeframe. Vyvgart and batoclimab data represent AChR+ patients, nipocalimab data represents all seropositive patients (including AChR+, MuSK+, and LRP4+). QW: Once Weekly; Q2W: Bi-weekly; LD: Loading Dose; AChR+: Acetylcholine receptor antibody-positive; MG-ADL: Myasthenia Gravis Activities of Daily Living scale. Figure reflects cross-trial comparisons and not data from head-to-head studies. Differences exist between trial designs and participant characteristics and caution should be exercised when comparing data across trials.
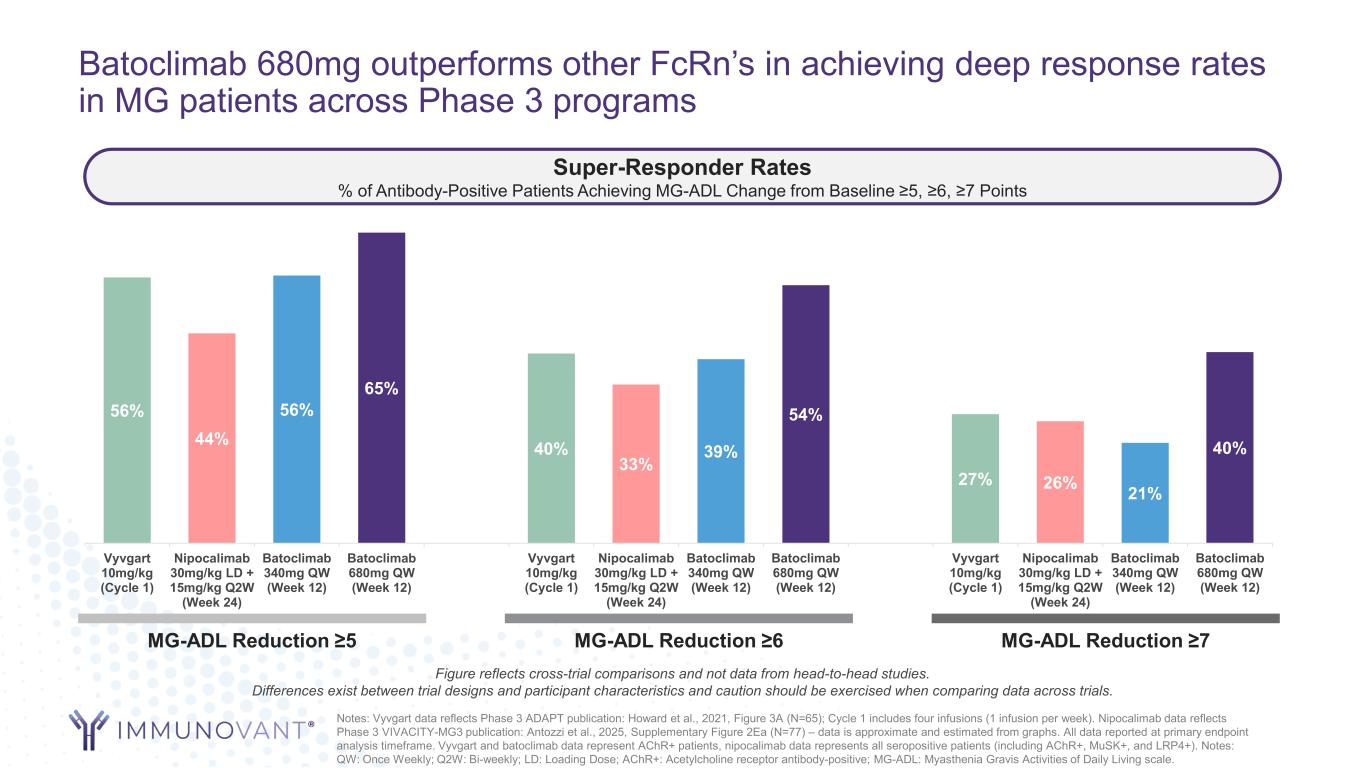
Batoclimab 680mg outperforms other FcRn’s in achieving deep response rates in MG patients across Phase 3 programs 56% 44% 56% 65% 40% 33% 39% 54% 27% 26% 21% 40% Vyvgart 10mg/kg (Cycle 1) Nipocalimab 30mg/kg LD + 15mg/kg Q2W (Week 24) Batoclimab 340mg QW (Week 12) Batoclimab 680mg QW (Week 12) Vyvgart 10mg/kg (Cycle 1) Nipocalimab 30mg/kg LD + 15mg/kg Q2W (Week 24) Batoclimab 340mg QW (Week 12) Batoclimab 680mg QW (Week 12) Vyvgart 10mg/kg (Cycle 1) Nipocalimab 30mg/kg LD + 15mg/kg Q2W (Week 24) Batoclimab 340mg QW (Week 12) Batoclimab 680mg QW (Week 12) Notes: Vyvgart data reflects Phase 3 ADAPT publication: Howard et al., 2021, Figure 3A (N=65); Cycle 1 includes four infusions (1 infusion per week). Nipocalimab data reflects Phase 3 VIVACITY-MG3 publication: Antozzi et al., 2025, Supplementary Figure 2Ea (N=77) – data is approximate and estimated from graphs. All data reported at primary endpoint analysis timeframe. Vyvgart and batoclimab data represent AChR+ patients, nipocalimab data represents all seropositive patients (including AChR+, MuSK+, and LRP4+). Notes: QW: Once Weekly; Q2W: Bi-weekly; LD: Loading Dose; AChR+: Acetylcholine receptor antibody-positive; MG-ADL: Myasthenia Gravis Activities of Daily Living scale. Super-Responder Rates % of Antibody-Positive Patients Achieving MG-ADL Change from Baseline ≥5, ≥6, ≥7 Points MG-ADL Reduction ≥7 MG-ADL Reduction ≥6 MG-ADL Reduction ≥5 Figure reflects cross-trial comparisons and not data from head-to-head studies. Differences exist between trial designs and participant characteristics and caution should be exercised when comparing data across trials.
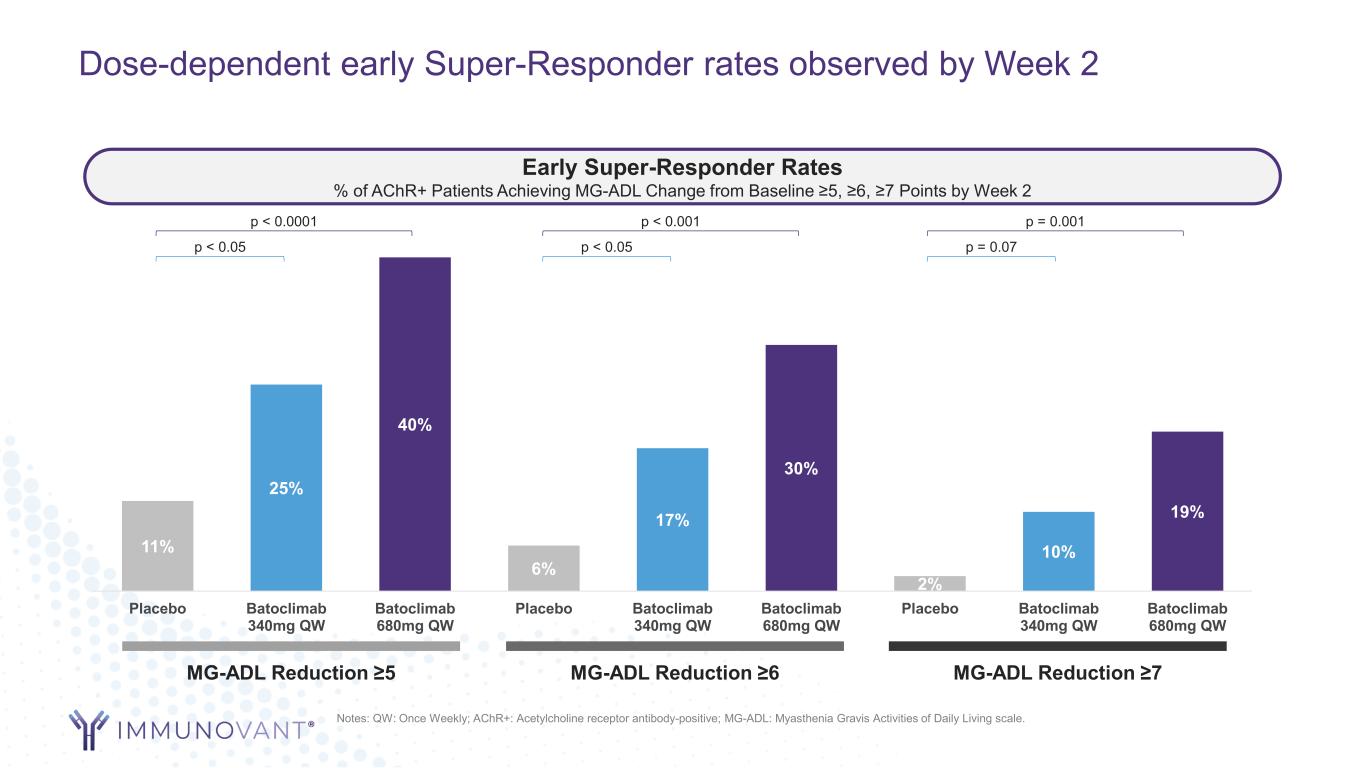
Dose-dependent early Super-Responder rates observed by Week 2 11% 25% 40% 6% 17% 30% 2% 10% 19% Placebo Batoclimab 340mg QW Batoclimab 680mg QW Placebo Batoclimab 340mg QW Batoclimab 680mg QW Placebo Batoclimab 340mg QW Batoclimab 680mg QW Notes: QW: Once Weekly; AChR+: Acetylcholine receptor antibody-positive; MG-ADL: Myasthenia Gravis Activities of Daily Living scale. Early Super-Responder Rates % of AChR+ Patients Achieving MG-ADL Change from Baseline ≥5, ≥6, ≥7 Points by Week 2 MG-ADL Reduction ≥5 MG-ADL Reduction ≥6 MG-ADL Reduction ≥7 p < 0.0001 p < 0.05 p < 0.001 p < 0.05 p = 0.001 p = 0.07
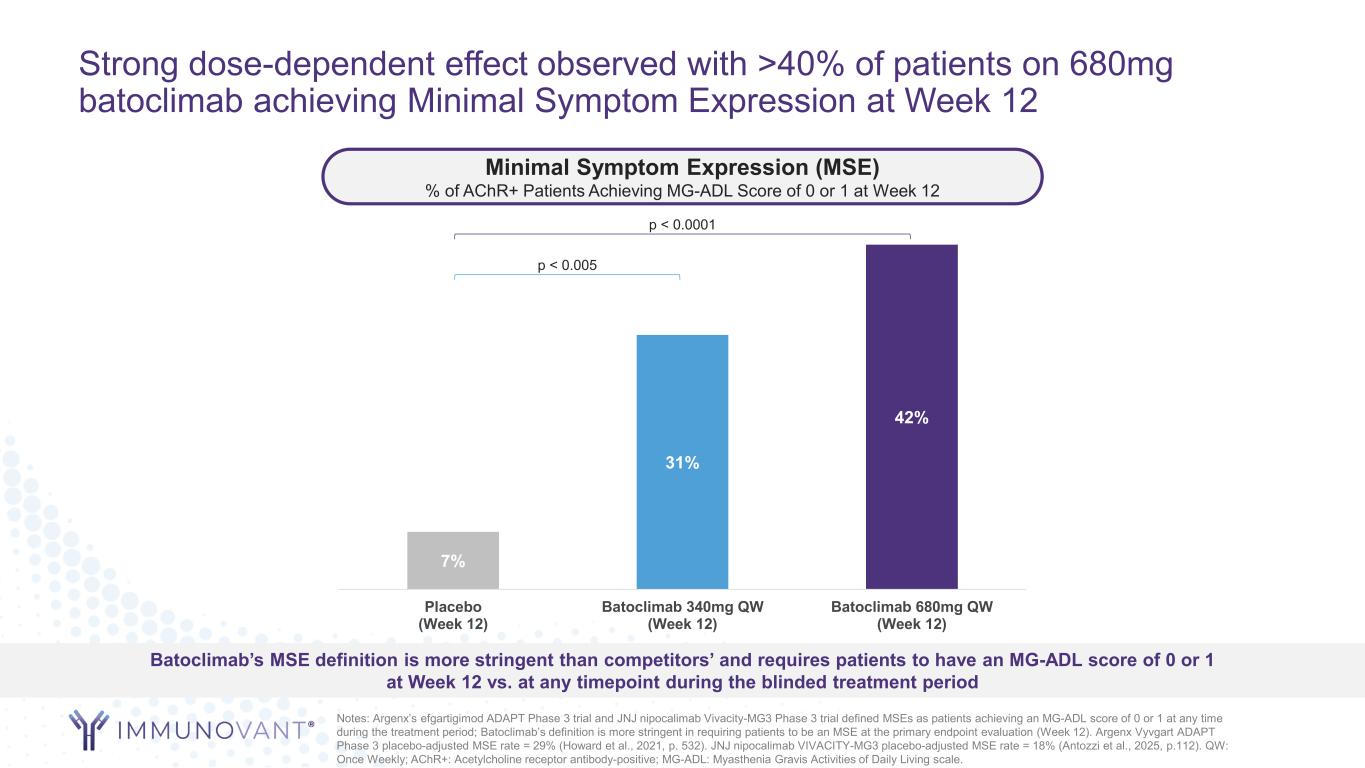
Strong dose-dependent effect observed with >40% of patients on 680mg batoclimab achieving Minimal Symptom Expression at Week 12 7% 31% 42% Placebo (Week 12) Batoclimab 340mg QW (Week 12) Batoclimab 680mg QW (Week 12) Notes: Argenx’s efgartigimod ADAPT Phase 3 trial and JNJ nipocalimab Vivacity-MG3 Phase 3 trial defined MSEs as patients achieving an MG-ADL score of 0 or 1 at any time during the treatment period; Batoclimab’s definition is more stringent in requiring patients to be an MSE at the primary endpoint evaluation (Week 12). Argenx Vyvgart ADAPT Phase 3 placebo-adjusted MSE rate = 29% (Howard et al., 2021, p. 532). JNJ nipocalimab VIVACITY-MG3 placebo-adjusted MSE rate = 18% (Antozzi et al., 2025, p.112). QW: Once Weekly; AChR+: Acetylcholine receptor antibody-positive; MG-ADL: Myasthenia Gravis Activities of Daily Living scale. Minimal Symptom Expression (MSE) % of AChR+ Patients Achieving MG-ADL Score of 0 or 1 at Week 12 Batoclimab’s MSE definition is more stringent than competitors’ and requires patients to have an MG-ADL score of 0 or 1 at Week 12 vs. at any timepoint during the blinded treatment period p < 0.0001 p < 0.005
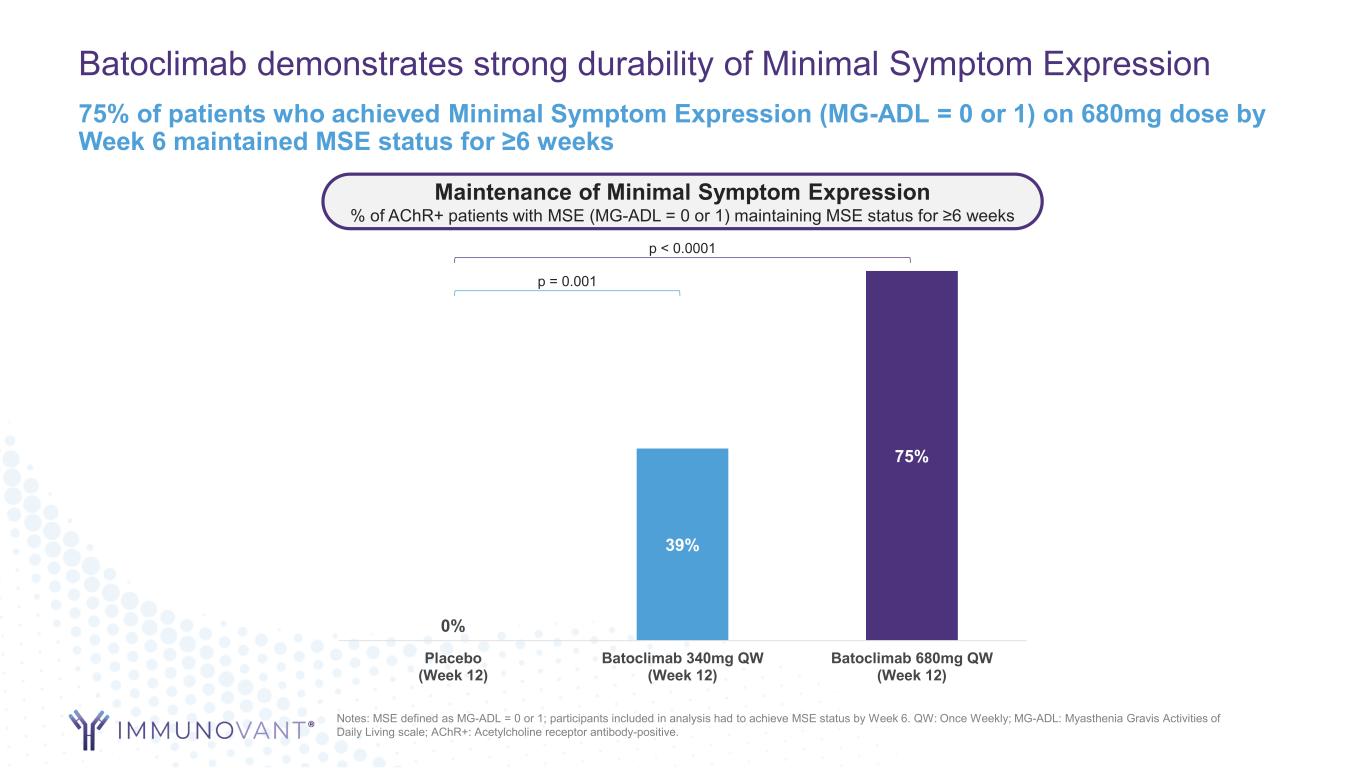
Batoclimab demonstrates strong durability of Minimal Symptom Expression 0% 39% 75% Placebo (Week 12) Batoclimab 340mg QW (Week 12) Batoclimab 680mg QW (Week 12) 75% of patients who achieved Minimal Symptom Expression (MG-ADL = 0 or 1) on 680mg dose by Week 6 maintained MSE status for ≥6 weeks Notes: MSE defined as MG-ADL = 0 or 1; participants included in analysis had to achieve MSE status by Week 6. QW: Once Weekly; MG-ADL: Myasthenia Gravis Activities of Daily Living scale; AChR+: Acetylcholine receptor antibody-positive. Maintenance of Minimal Symptom Expression % of AChR+ patients with MSE (MG-ADL = 0 or 1) maintaining MSE status for ≥6 weeks p < 0.0001 p = 0.001
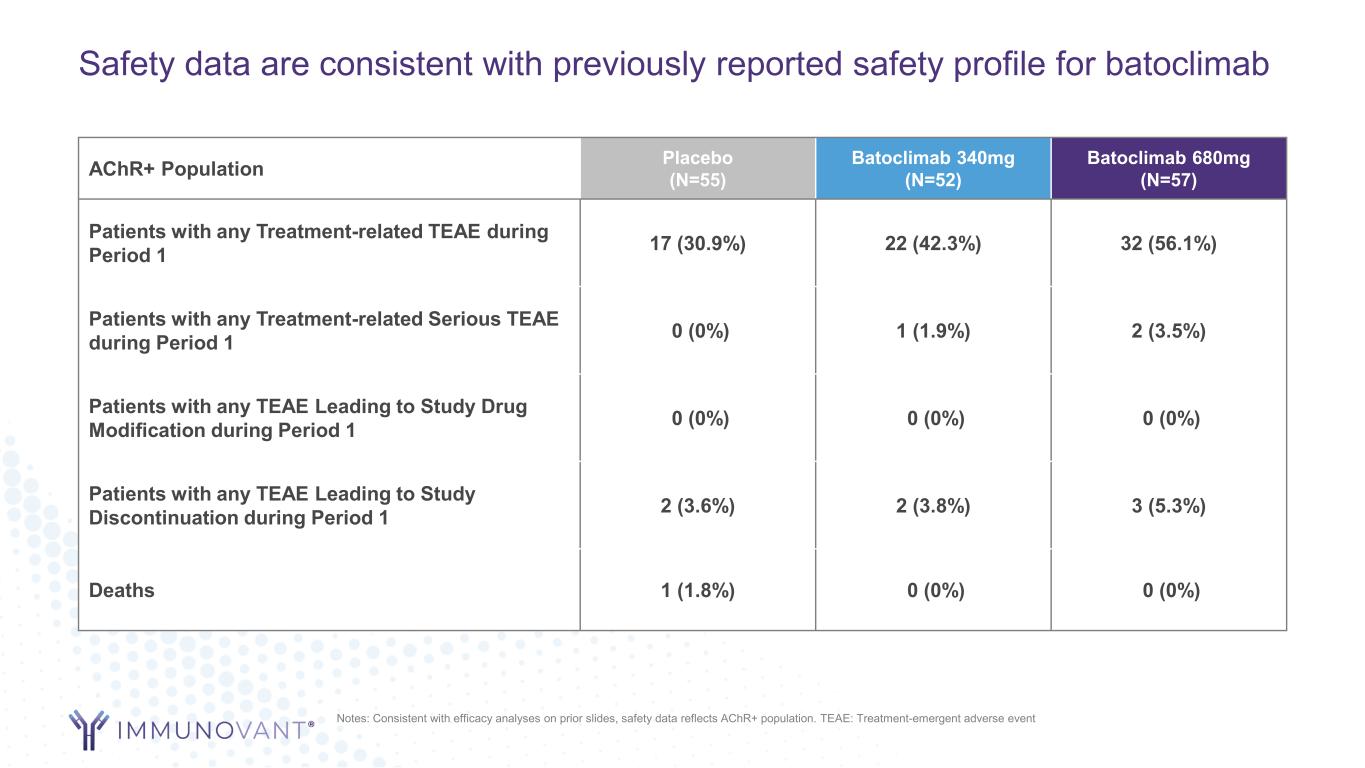
Safety data are consistent with previously reported safety profile for batoclimab AChR+ Population Placebo (N=55) Batoclimab 340mg (N=52) Batoclimab 680mg (N=57) Patients with any Treatment-related TEAE during Period 1 17 (30.9%) 22 (42.3%) 32 (56.1%) Patients with any Treatment-related Serious TEAE during Period 1 0 (0%) 1 (1.9%) 2 (3.5%) Patients with any TEAE Leading to Study Drug Modification during Period 1 0 (0%) 0 (0%) 0 (0%) Patients with any TEAE Leading to Study Discontinuation during Period 1 2 (3.6%) 2 (3.8%) 3 (5.3%) Deaths 1 (1.8%) 0 (0%) 0 (0%) Notes: Consistent with efficacy analyses on prior slides, safety data reflects AChR+ population. TEAE: Treatment-emergent adverse event
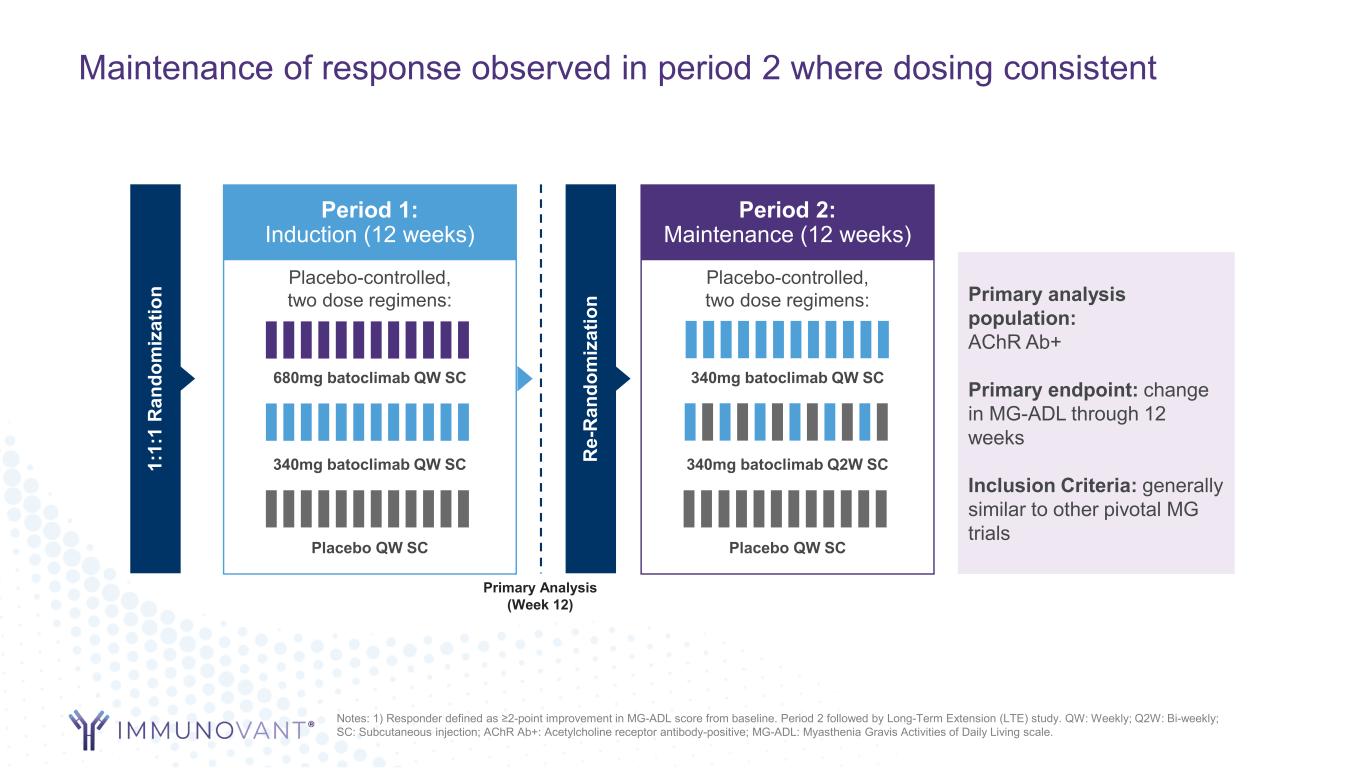
Maintenance of response observed in period 2 where dosing consistent Notes: 1) Responder defined as ≥2-point improvement in MG-ADL score from baseline. Period 2 followed by Long-Term Extension (LTE) study. QW: Weekly; Q2W: Bi-weekly; SC: Subcutaneous injection; AChR Ab+: Acetylcholine receptor antibody-positive; MG-ADL: Myasthenia Gravis Activities of Daily Living scale. Period 1: Induction (12 weeks) 1: 1: 1 R an do m iz at io n Placebo-controlled, two dose regimens: Placebo QW SC 340mg batoclimab QW SC 680mg batoclimab QW SC Period 2: Maintenance (12 weeks) R e- R an do m iz at io n Placebo-controlled, two dose regimens: Placebo QW SC 340mg batoclimab Q2W SC 340mg batoclimab QW SC Primary analysis population: AChR Ab+ Primary endpoint: change in MG-ADL through 12 weeks Inclusion Criteria: generally similar to other pivotal MG trials Primary Analysis (Week 12)

CIDP Initial Period 1 Combined Results
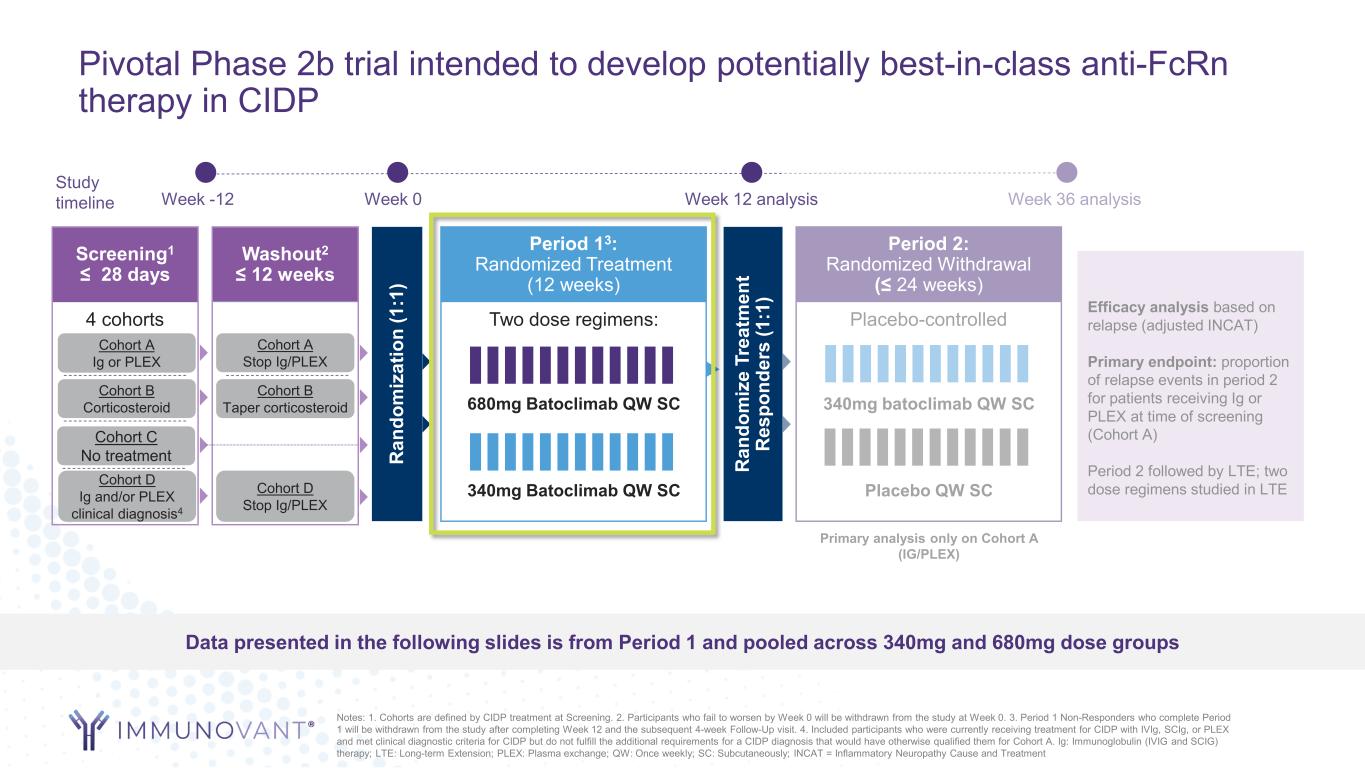
Pivotal Phase 2b trial intended to develop potentially best-in-class anti-FcRn therapy in CIDP Screening1 ≤ 28 days 4 cohorts Week -12 Week 0 Week 36 analysis Study timeline Cohort A Ig or PLEX Cohort B Corticosteroid Cohort C No treatment Washout2 ≤ 12 weeks Cohort A Stop Ig/PLEX Cohort B Taper corticosteroid R an do m iz at io n (1 :1 ) R an do m iz e Tr ea tm en t R es po nd er s (1 :1 ) Period 13: Randomized Treatment (12 weeks) Two dose regimens: 340mg Batoclimab QW SC 680mg Batoclimab QW SC Period 2: Randomized Withdrawal (≤ 24 weeks) Placebo-controlled Placebo QW SC 340mg batoclimab QW SC Efficacy analysis based on relapse (adjusted INCAT) Primary endpoint: proportion of relapse events in period 2 for patients receiving Ig or PLEX at time of screening (Cohort A) Period 2 followed by LTE; two dose regimens studied in LTE Primary analysis only on Cohort A (IG/PLEX) Cohort D Ig and/or PLEX clinical diagnosis4 Cohort D Stop Ig/PLEX Data presented in the following slides is from Period 1 and pooled across 340mg and 680mg dose groups Week 12 analysis Notes: 1. Cohorts are defined by CIDP treatment at Screening. 2. Participants who fail to worsen by Week 0 will be withdrawn from the study at Week 0. 3. Period 1 Non-Responders who complete Period 1 will be withdrawn from the study after completing Week 12 and the subsequent 4-week Follow-Up visit. 4. Included participants who were currently receiving treatment for CIDP with IVIg, SCIg, or PLEX and met clinical diagnostic criteria for CIDP but do not fulfill the additional requirements for a CIDP diagnosis that would have otherwise qualified them for Cohort A. Ig: Immunoglobulin (IVIG and SCIG) therapy; LTE: Long-term Extension; PLEX: Plasma exchange; QW: Once weekly; SC: Subcutaneously; INCAT = Inflammatory Neuropathy Cause and Treatment
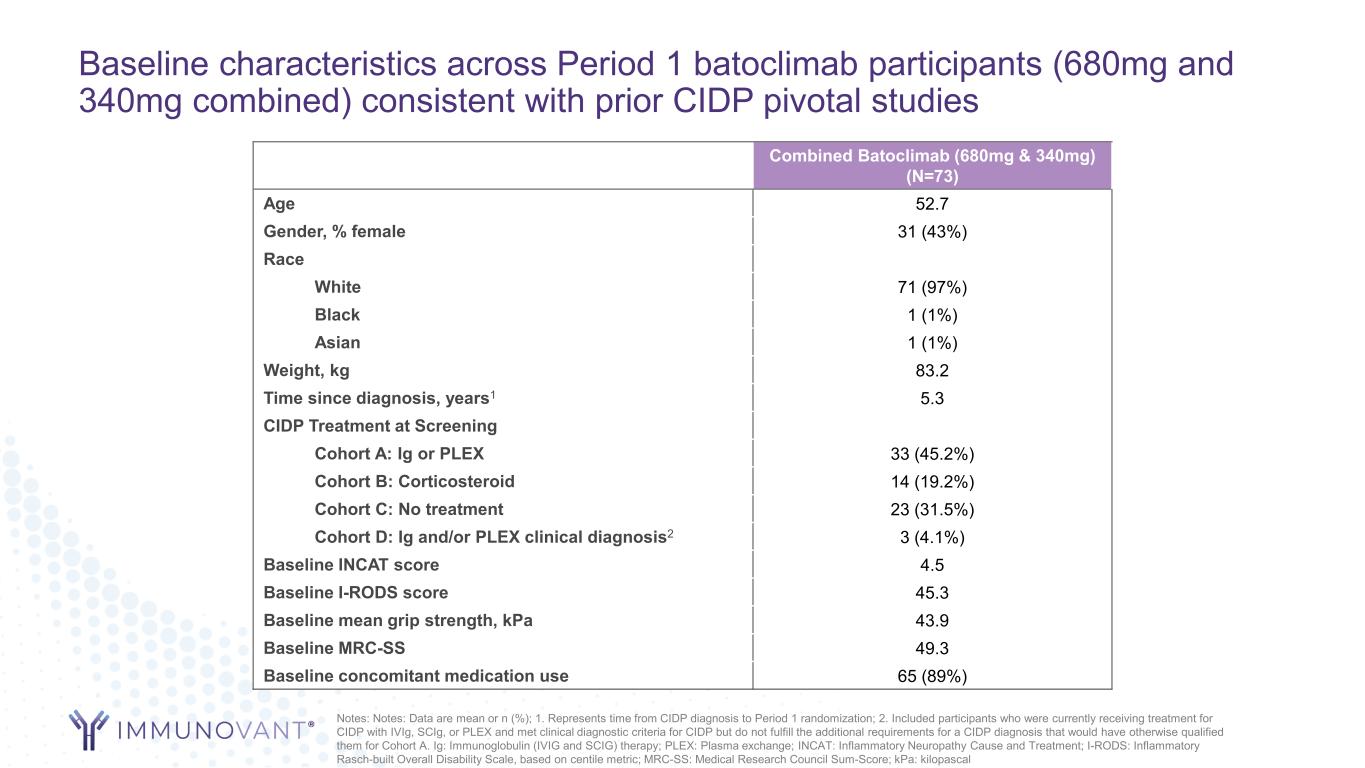
Baseline characteristics across Period 1 batoclimab participants (680mg and 340mg combined) consistent with prior CIDP pivotal studies Notes: Notes: Data are mean or n (%); 1. Represents time from CIDP diagnosis to Period 1 randomization; 2. Included participants who were currently receiving treatment for CIDP with IVIg, SCIg, or PLEX and met clinical diagnostic criteria for CIDP but do not fulfill the additional requirements for a CIDP diagnosis that would have otherwise qualified them for Cohort A. Ig: Immunoglobulin (IVIG and SCIG) therapy; PLEX: Plasma exchange; INCAT: Inflammatory Neuropathy Cause and Treatment; I-RODS: Inflammatory Rasch-built Overall Disability Scale, based on centile metric; MRC-SS: Medical Research Council Sum-Score; kPa: kilopascal Combined Batoclimab (680mg & 340mg) (N=73) Age 52.7 Gender, % female 31 (43%) Race White 71 (97%) Black 1 (1%) Asian 1 (1%) Weight, kg 83.2 Time since diagnosis, years1 5.3 CIDP Treatment at Screening Cohort A: Ig or PLEX 33 (45.2%) Cohort B: Corticosteroid 14 (19.2%) Cohort C: No treatment 23 (31.5%) Cohort D: Ig and/or PLEX clinical diagnosis2 3 (4.1%) Baseline INCAT score 4.5 Baseline I-RODS score 45.3 Baseline mean grip strength, kPa 43.9 Baseline MRC-SS 49.3 Baseline concomitant medication use 65 (89%)
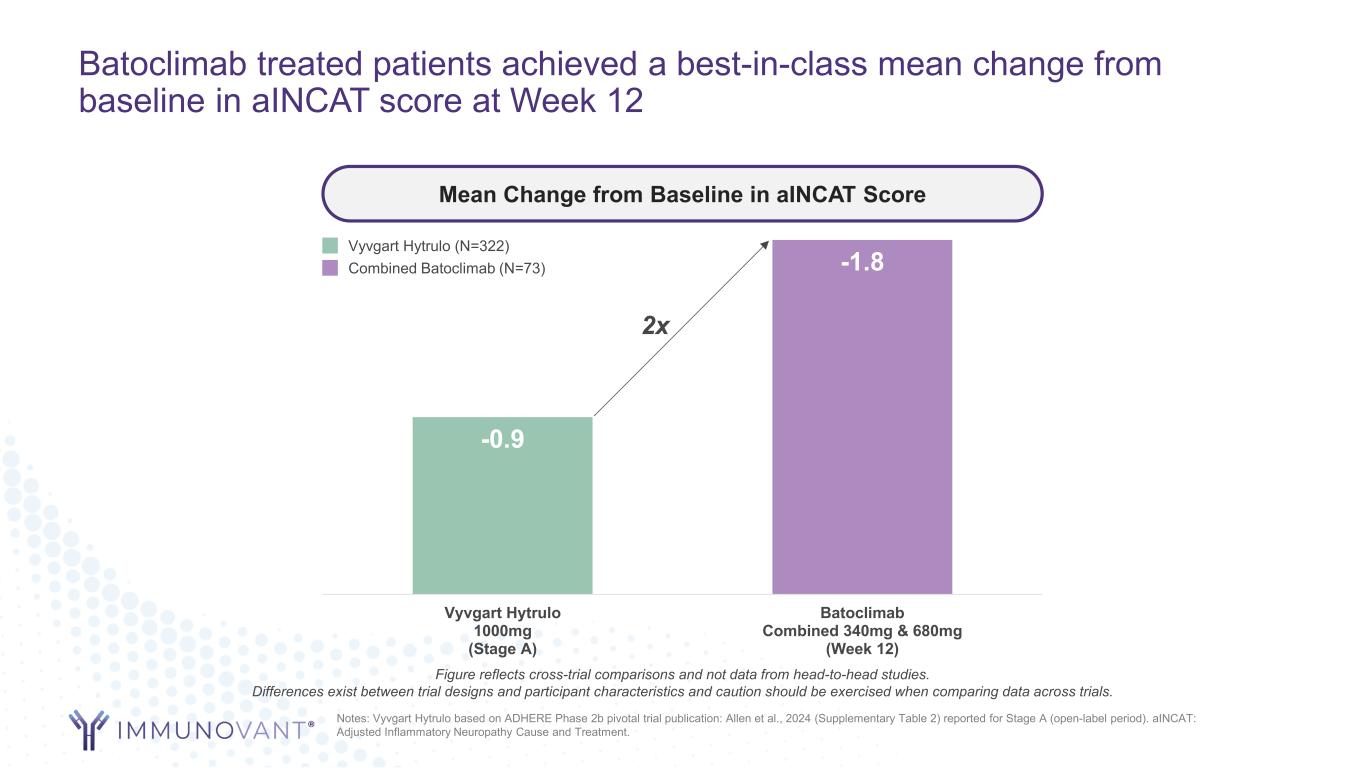
Batoclimab treated patients achieved a best-in-class mean change from baseline in aINCAT score at Week 12 -0.9 -1.8 Vyvgart Hytrulo 1000mg (Stage A) Batoclimab Combined 340mg & 680mg (Week 12) Mean Change from Baseline in aINCAT Score Notes: Vyvgart Hytrulo based on ADHERE Phase 2b pivotal trial publication: Allen et al., 2024 (Supplementary Table 2) reported for Stage A (open-label period). aINCAT: Adjusted Inflammatory Neuropathy Cause and Treatment. 2x Vyvgart Hytrulo (N=322) Combined Batoclimab (N=73) Figure reflects cross-trial comparisons and not data from head-to-head studies. Differences exist between trial designs and participant characteristics and caution should be exercised when comparing data across trials.
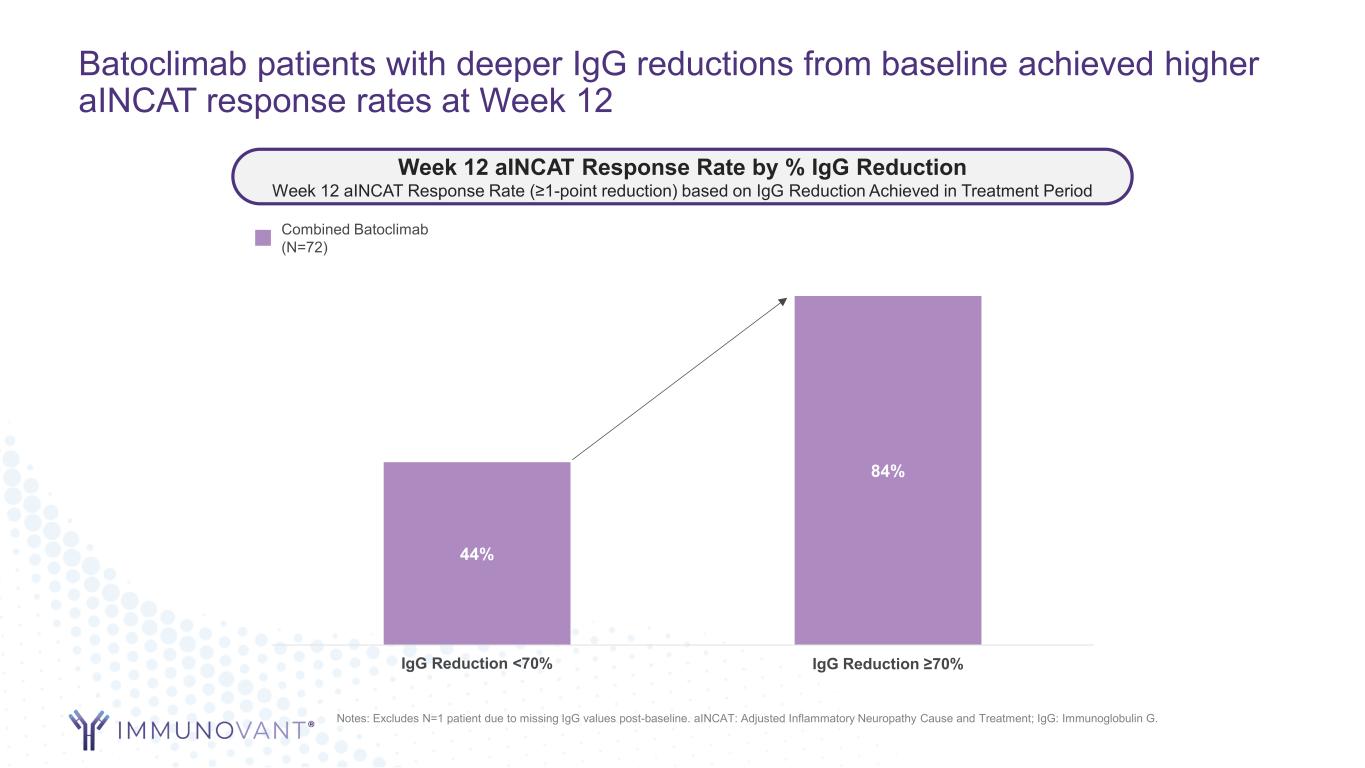
Batoclimab patients with deeper IgG reductions from baseline achieved higher aINCAT response rates at Week 12 44% 84% IgG Reduction <70% IgG Reduction ≥70% Notes: Excludes N=1 patient due to missing IgG values post-baseline. aINCAT: Adjusted Inflammatory Neuropathy Cause and Treatment; IgG: Immunoglobulin G. Week 12 aINCAT Response Rate by % IgG Reduction Week 12 aINCAT Response Rate (≥1-point reduction) based on IgG Reduction Achieved in Treatment Period Combined Batoclimab (N=72)
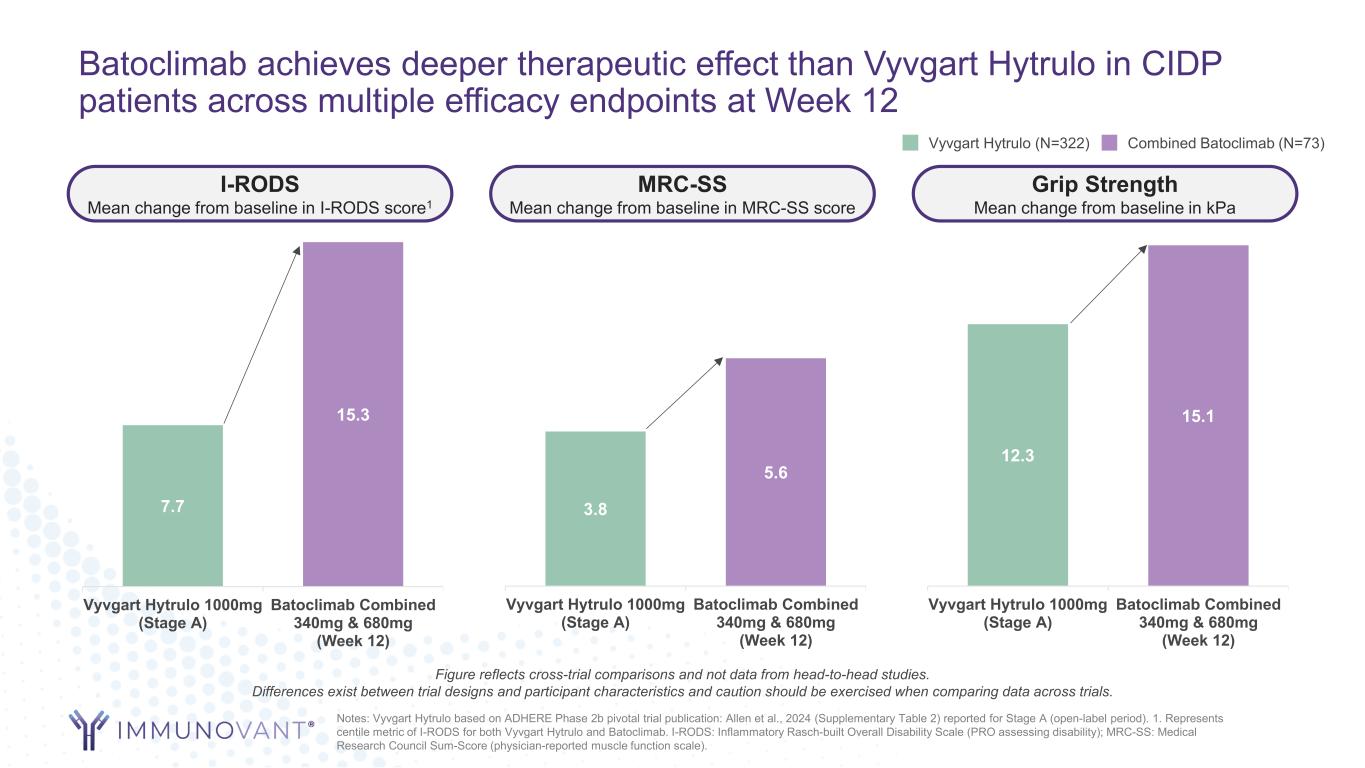
Batoclimab achieves deeper therapeutic effect than Vyvgart Hytrulo in CIDP patients across multiple efficacy endpoints at Week 12 7.7 15.3 Vyvgart Hytrulo 1000mg (Stage A) Batoclimab Combined 340mg & 680mg (Week 12) 3.8 5.6 Vyvgart Hytrulo 1000mg (Stage A) Batoclimab Combined 340mg & 680mg (Week 12) 12.3 15.1 Vyvgart Hytrulo 1000mg (Stage A) Batoclimab Combined 340mg & 680mg (Week 12) I-RODS Mean change from baseline in I-RODS score1 MRC-SS Mean change from baseline in MRC-SS score Grip Strength Mean change from baseline in kPa Notes: Vyvgart Hytrulo based on ADHERE Phase 2b pivotal trial publication: Allen et al., 2024 (Supplementary Table 2) reported for Stage A (open-label period). 1. Represents centile metric of I-RODS for both Vyvgart Hytrulo and Batoclimab. I-RODS: Inflammatory Rasch-built Overall Disability Scale (PRO assessing disability); MRC-SS: Medical Research Council Sum-Score (physician-reported muscle function scale). Vyvgart Hytrulo (N=322) Combined Batoclimab (N=73) Figure reflects cross-trial comparisons and not data from head-to-head studies. Differences exist between trial designs and participant characteristics and caution should be exercised when comparing data across trials.

Lower is Better
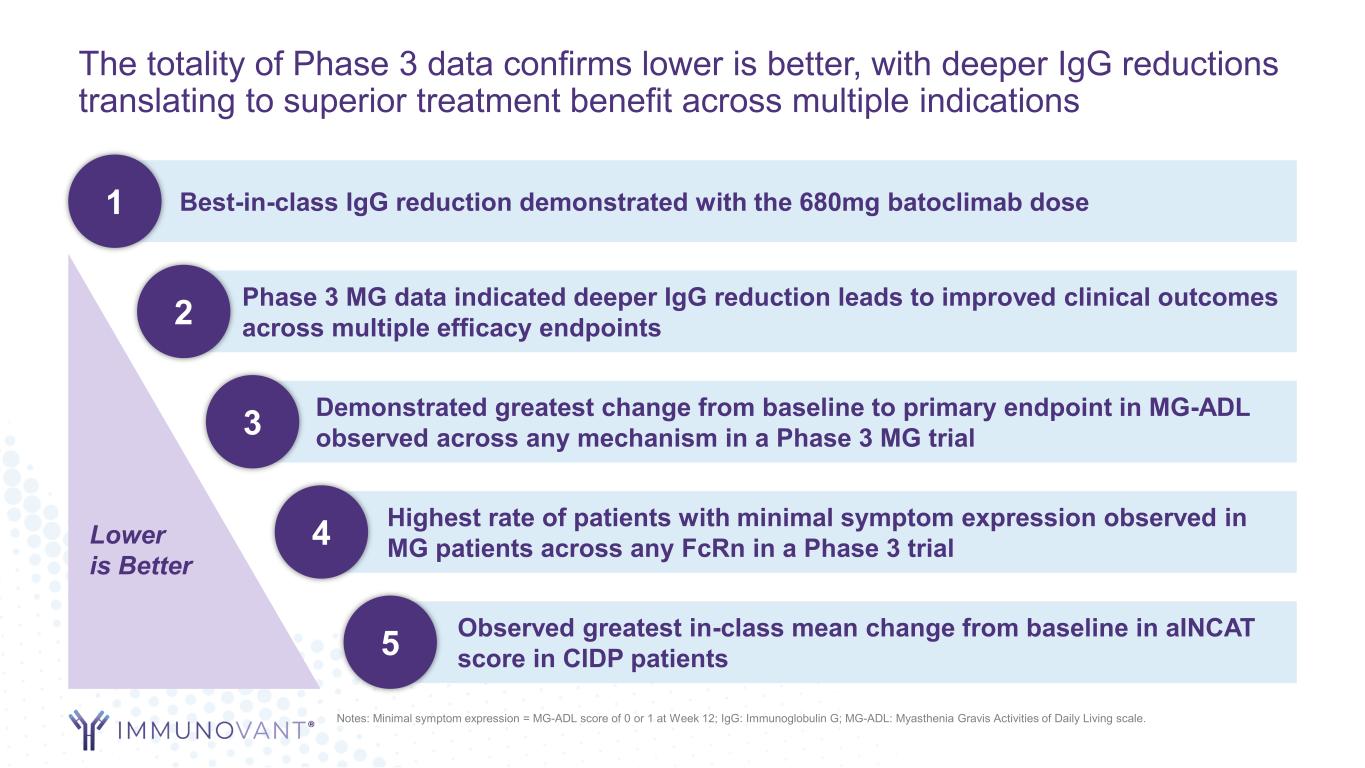
The totality of Phase 3 data confirms lower is better, with deeper IgG reductions translating to superior treatment benefit across multiple indications Best-in-class IgG reduction demonstrated with the 680mg batoclimab dose1 Phase 3 MG data indicated deeper IgG reduction leads to improved clinical outcomes across multiple efficacy endpoints2 Demonstrated greatest change from baseline to primary endpoint in MG-ADL observed across any mechanism in a Phase 3 MG trial3 Highest rate of patients with minimal symptom expression observed in MG patients across any FcRn in a Phase 3 trial4Lower is Better Notes: Minimal symptom expression = MG-ADL score of 0 or 1 at Week 12; IgG: Immunoglobulin G; MG-ADL: Myasthenia Gravis Activities of Daily Living scale. Observed greatest in-class mean change from baseline in aINCAT score in CIDP patients 5
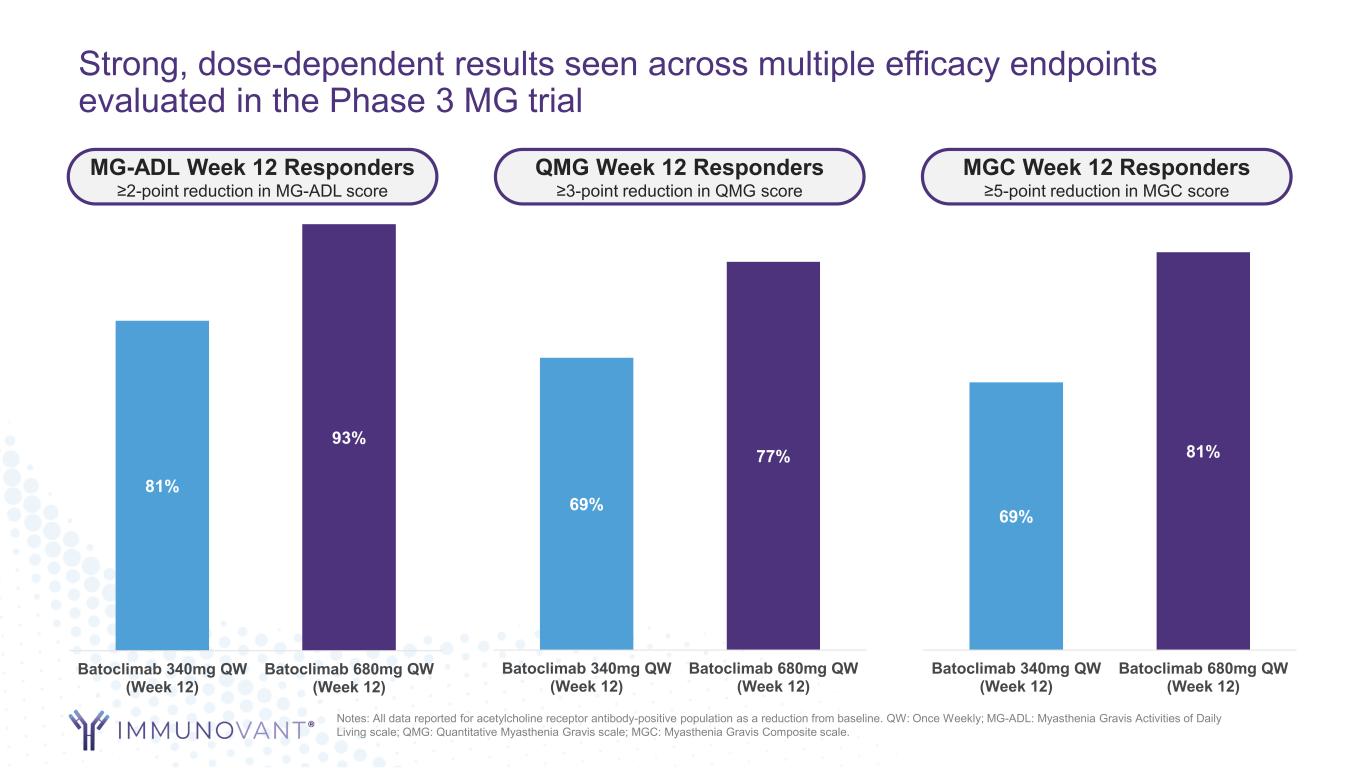
Strong, dose-dependent results seen across multiple efficacy endpoints evaluated in the Phase 3 MG trial Notes: All data reported for acetylcholine receptor antibody-positive population as a reduction from baseline. QW: Once Weekly; MG-ADL: Myasthenia Gravis Activities of Daily Living scale; QMG: Quantitative Myasthenia Gravis scale; MGC: Myasthenia Gravis Composite scale. 69% 77% Batoclimab 340mg QW (Week 12) Batoclimab 680mg QW (Week 12) 69% 81% Batoclimab 340mg QW (Week 12) Batoclimab 680mg QW (Week 12) 81% 93% Batoclimab 340mg QW (Week 12) Batoclimab 680mg QW (Week 12) QMG Week 12 Responders ≥3-point reduction in QMG score MGC Week 12 Responders ≥5-point reduction in MGC score MG-ADL Week 12 Responders ≥2-point reduction in MG-ADL score
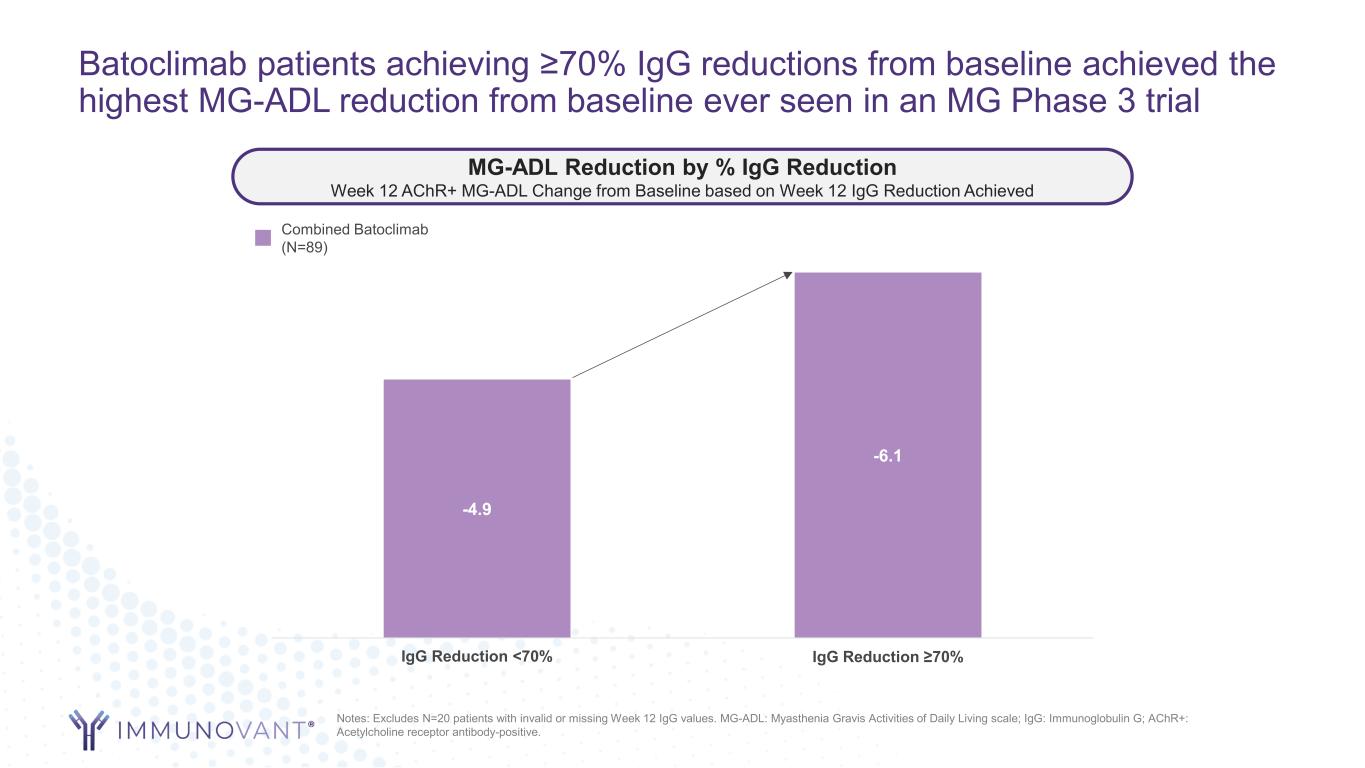
Batoclimab patients achieving ≥70% IgG reductions from baseline achieved the highest MG-ADL reduction from baseline ever seen in an MG Phase 3 trial -4.9 -6.1 IgG Reduction <70% IgG Reduction ≥70% Combined Batoclimab (N=89) Notes: Excludes N=20 patients with invalid or missing Week 12 IgG values. MG-ADL: Myasthenia Gravis Activities of Daily Living scale; IgG: Immunoglobulin G; AChR+: Acetylcholine receptor antibody-positive. MG-ADL Reduction by % IgG Reduction Week 12 AChR+ MG-ADL Change from Baseline based on Week 12 IgG Reduction Achieved
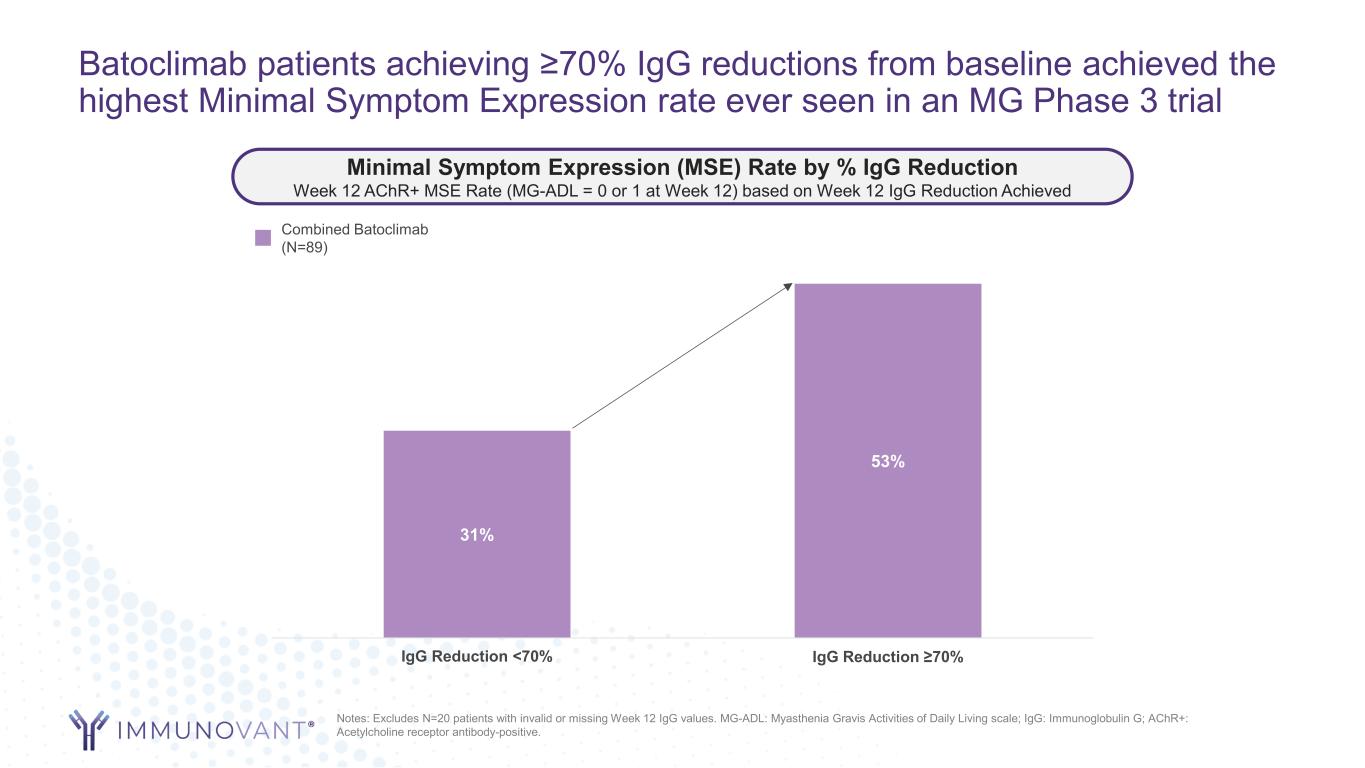
Batoclimab patients achieving ≥70% IgG reductions from baseline achieved the highest Minimal Symptom Expression rate ever seen in an MG Phase 3 trial 31% 53% IgG Reduction <70% IgG Reduction ≥70% Notes: Excludes N=20 patients with invalid or missing Week 12 IgG values. MG-ADL: Myasthenia Gravis Activities of Daily Living scale; IgG: Immunoglobulin G; AChR+: Acetylcholine receptor antibody-positive. Minimal Symptom Expression (MSE) Rate by % IgG Reduction Week 12 AChR+ MSE Rate (MG-ADL = 0 or 1 at Week 12) based on Week 12 IgG Reduction Achieved Combined Batoclimab (N=89)
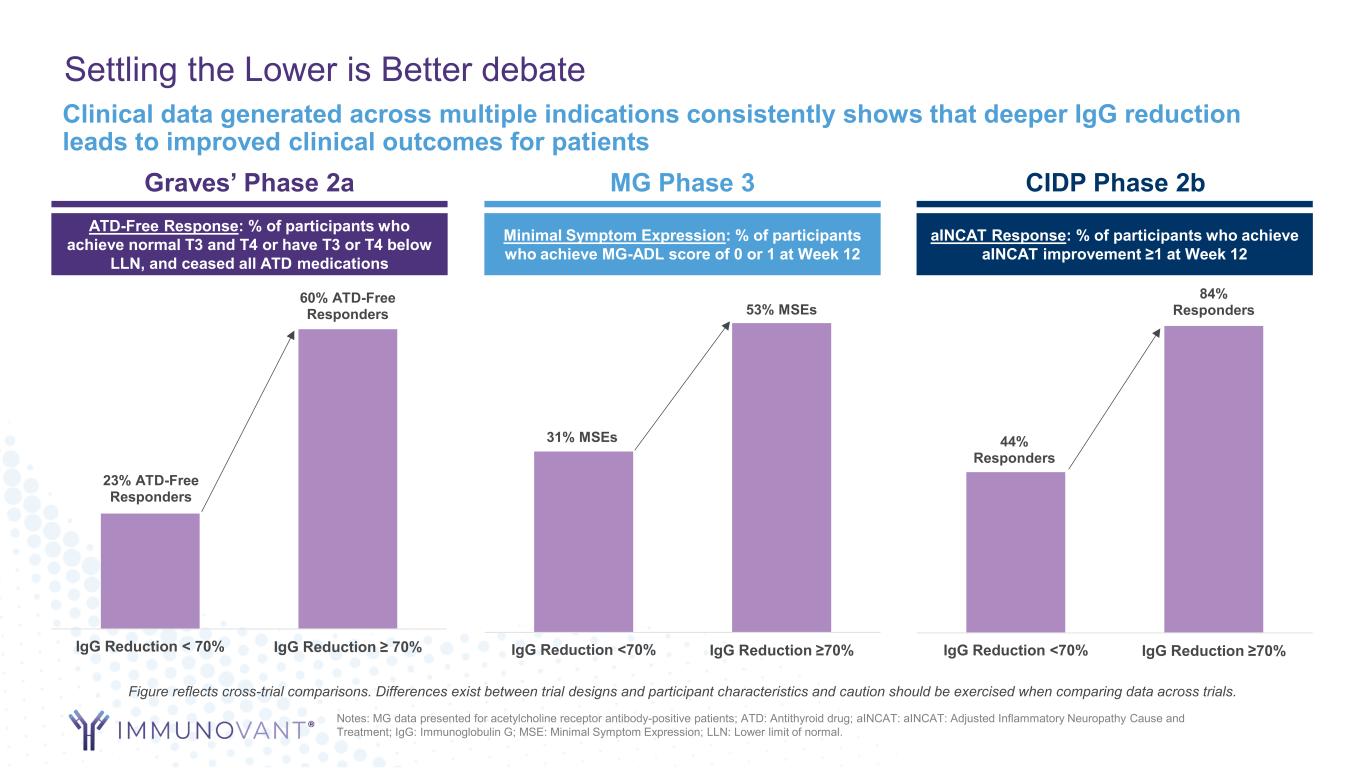
Settling the Lower is Better debate Graves’ Phase 2a MG Phase 3 CIDP Phase 2b 23% ATD-Free Responders 60% ATD-Free Responders IgG Reduction < 70% IgG Reduction ≥ 70% ATD-Free Response: % of participants who achieve normal T3 and T4 or have T3 or T4 below LLN, and ceased all ATD medications Minimal Symptom Expression: % of participants who achieve MG-ADL score of 0 or 1 at Week 12 31% MSEs 53% MSEs IgG Reduction <70% IgG Reduction ≥70% aINCAT Response: % of participants who achieve aINCAT improvement ≥1 at Week 12 44% Responders 84% Responders IgG Reduction <70% IgG Reduction ≥70% Clinical data generated across multiple indications consistently shows that deeper IgG reduction leads to improved clinical outcomes for patients Notes: MG data presented for acetylcholine receptor antibody-positive patients; ATD: Antithyroid drug; aINCAT: aINCAT: Adjusted Inflammatory Neuropathy Cause and Treatment; IgG: Immunoglobulin G; MSE: Minimal Symptom Expression; LLN: Lower limit of normal. Figure reflects cross-trial comparisons. Differences exist between trial designs and participant characteristics and caution should be exercised when comparing data across trials.

Path Forward in MG with IMVT-1402 Immunovant does not plan to seek regulatory approval for batoclimab in MG or CIDP at present

MG patients and providers indicate a need for deeper and more durable disease control Neurologists agree that despite recent advancements with FcRn inhibitors, there is room for greater disease control (e.g., deeper responses)195% Neurologists report that their patients experience breakthrough symptoms with currently available FcRn inhibitors1 84% Neurologists indicate that their existing MG patients could benefit from a new therapy that offers greater durability2 95% Notes: 1. IMVT Market Research HCP MG Unmet Need: Part II (n=85), 2025 Neurologists/Neuromuscular Specialists treating ~28 gMG patients/month, reporting T3B percentages; 2: GMG treatment preferences survey 2024 (n=7), Neurologists/Neuromuscular Specialists treating ~38 gMG patients/year.
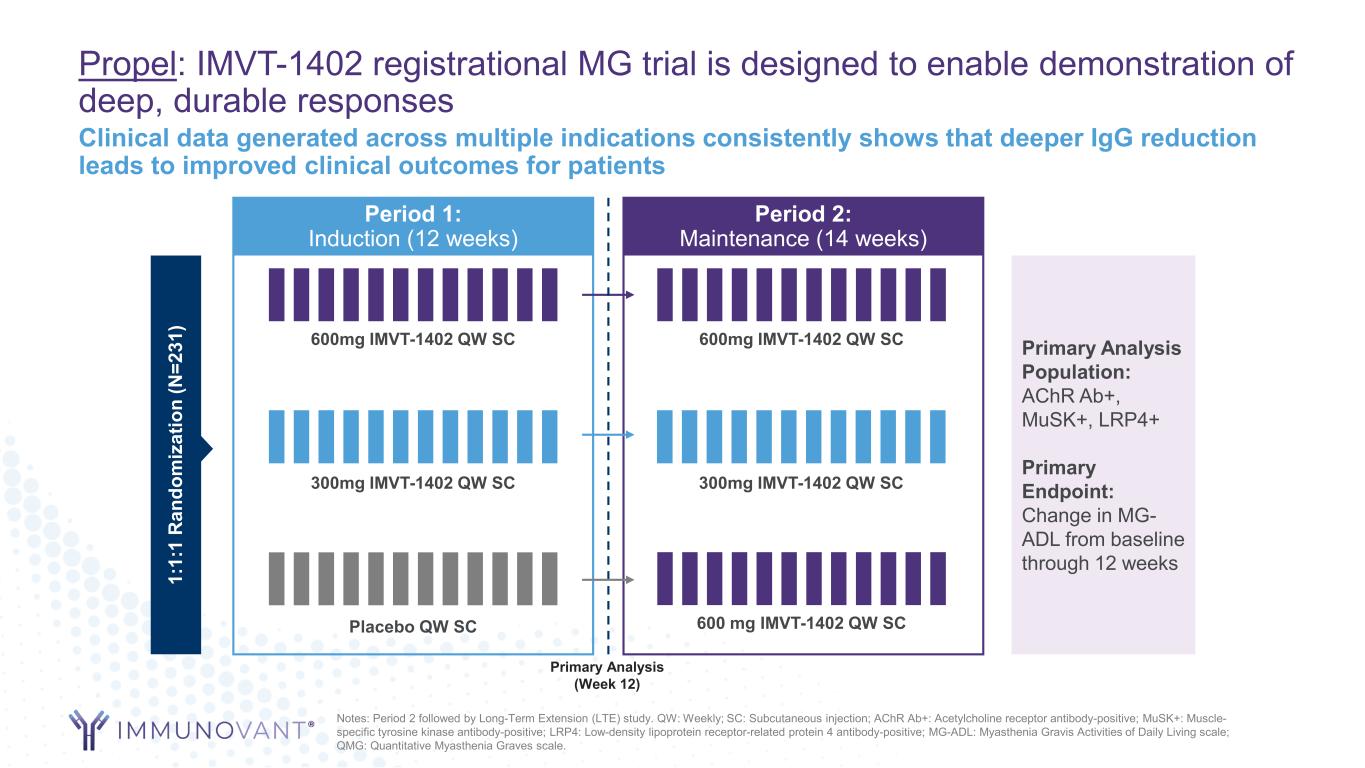
Propel: IMVT-1402 registrational MG trial is designed to enable demonstration of deep, durable responses Period 1: Induction (12 weeks) 1: 1: 1 R an do m iz at io n (N =2 31 ) Placebo QW SC 600mg IMVT-1402 QW SC Period 2: Maintenance (14 weeks) Primary Analysis Population: AChR Ab+, MuSK+, LRP4+ Primary Endpoint: Change in MG- ADL from baseline through 12 weeks Primary Analysis (Week 12) 300mg IMVT-1402 QW SC 600mg IMVT-1402 QW SC 300mg IMVT-1402 QW SC 600 mg IMVT-1402 QW SC Notes: Period 2 followed by Long-Term Extension (LTE) study. QW: Weekly; SC: Subcutaneous injection; AChR Ab+: Acetylcholine receptor antibody-positive; MuSK+: Muscle- specific tyrosine kinase antibody-positive; LRP4: Low-density lipoprotein receptor-related protein 4 antibody-positive; MG-ADL: Myasthenia Gravis Activities of Daily Living scale; QMG: Quantitative Myasthenia Graves scale. Clinical data generated across multiple indications consistently shows that deeper IgG reduction leads to improved clinical outcomes for patients

Concluding Thoughts: 1402 Positioned to be Best-in-Class
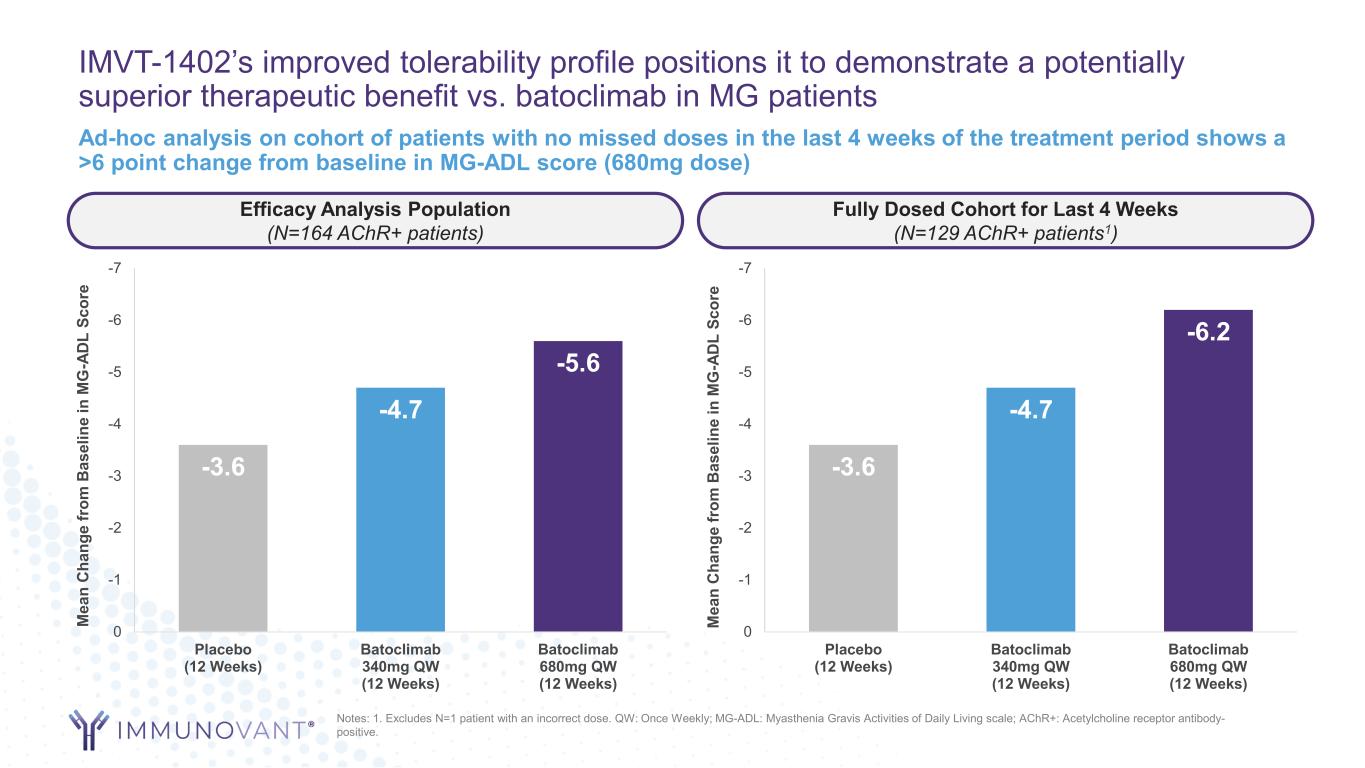
IMVT-1402’s improved tolerability profile positions it to demonstrate a potentially superior therapeutic benefit vs. batoclimab in MG patients Ad-hoc analysis on cohort of patients with no missed doses in the last 4 weeks of the treatment period shows a >6 point change from baseline in MG-ADL score (680mg dose) -3.6 -4.7 -5.6 -7 -6 -5 -4 -3 -2 -1 0 Placebo (12 Weeks) Batoclimab 340mg QW (12 Weeks) Batoclimab 680mg QW (12 Weeks) M ea n C ha ng e fr om B as el in e in M G -A D L Sc or e Efficacy Analysis Population (N=164 AChR+ patients) -3.6 -4.7 -6.2 -7 -6 -5 -4 -3 -2 -1 0 Placebo (12 Weeks) Batoclimab 340mg QW (12 Weeks) Batoclimab 680mg QW (12 Weeks) M ea n C ha ng e fr om B as el in e in M G -A D L Sc or e Fully Dosed Cohort for Last 4 Weeks (N=129 AChR+ patients1) Notes: 1. Excludes N=1 patient with an incorrect dose. QW: Once Weekly; MG-ADL: Myasthenia Gravis Activities of Daily Living scale; AChR+: Acetylcholine receptor antibody- positive.
Batoclimab data positions IMVT-1402 as potentially best-in-class FcRn and enables acceleration of IMVT-1402 registration programs in MG and CIDP Notes: *Not including any potential patent term extension; IP: Intellectual Property Novel, fully human, monoclonal antibody inhibiting FcRn-mediated recycling of IgG IMVT-1402 MG and CIDP data indicates greater IgG reduction correlates to improved clinical outcomes Learnings from batoclimab Phase 3 MG and CIDP programs will accelerate IMVT-1402’s registration programs in both indications IMVT-1402 is the only FcRn positioned to launch in an autoinjector as the commercial presentation for the first and all subsequent indications Long IP run-time with issued composition of matter patent to 2043*