
Targeted science, Tailored solutions for people with autoimmune disease Portfolio Update November 28, 2023 Exhibit 99.2

Forward-Looking Statements 2 This presentation contains forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. The use of words such as "can," “may,” “might,” “will,” “would,” “should,” “expect,” “believe,” “estimate,” “design,” “plan,” "intend," and other similar expressions are intended to identify forward-looking statements. Such forward looking statements include the timing and results of Immunovant’s clinical trials of IMVT–1402; expectations with respect to these planned clinical trials; the size and growth of the potential markets for Immunovant's product candidates and indication selections; Immunovant's beliefs regarding the potential benefits of IMVT-1402's unique product attributes including IMVT-1402's potential best-in-class Immunoglobulin G (IgG) reduction and tolerability and IMVT-1402's potential as a treatment for chronic inflammatory demyelinating polyneuropathy, Graves' disease, rheumatoid arthritis, and other autoimmune diseases; whether, if approved, IMVT-1402 will be successfully distributed, marketed, and commercialized; and Immunovant's expectations regarding the issuance and term of any pending patents. All forward-looking statements are based on estimates and assumptions by Immunovant’s management that, although Immunovant believes to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that Immunovant expected. Such risks and uncertainties include, among others: initial results or other preliminary analyses or results of early clinical trials may not be predictive of final trial results or of the results of later clinical trials; results of animal studies may not be predictive of results in humans; the timing and availability of data from clinical trials; the timing of discussions with regulatory agencies, as well as regulatory submissions and potential approvals; the continued development of Immunovant’s product candidates, including the timing of the commencement of additional clinical trials; Immunovant’s scientific approach, clinical trial design, indication selection, and general development progress; future clinical trials may not confirm any safety, potency, or other product characteristics described or assumed in this presentation; any product candidate that Immunovant develops may not progress through clinical development or receive required regulatory approvals within expected timelines or at all; Immunovant's pending composition of matter patent for IMVT-1402 may not be issued; Immunovant’s product candidates may not be beneficial to patients, or even if approved by regulatory authorities, successfully commercialized; the effect of global factors such as impacts from the post-COVID-19 environment, geopolitical tensions, and adverse macroeconomic conditions on Immunovant’s business operations and supply chains, including its clinical development plans and timelines; Immunovant’s business is heavily dependent on the successful development, regulatory approval and commercialization of batoclimab and IMVT-1402; Immunovant is at an early stage in development for IMVT-1402 and in various stages of clinical development for batoclimab; and Immunovant will require additional capital to fund its operations and advance batoclimab and IMVT-1402 through clinical development. These and other risks and uncertainties are more fully described in Immunovant’s periodic and other reports filed with the Securities and Exchange Commission (SEC), including in the section titled “Risk Factors” in Immunovant’s most recent Quarterly Report on Form 10-Q for the fiscal quarter ended September 30, 2023, filed with the SEC on November 9, 2023, and Immunovant’s subsequent filings with the SEC. Any forward-looking statement speaks only as of the date on which it was made. Immunovant undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. All trademarks, trade names, service marks, and copyrights appearing in this presentation are the property of their respective owners. Dates used in this presentation refer to the applicable calendar year unless otherwise noted.
Our Vision: Normal Lives for People with Autoimmune Disease 3 Love Trailblazing All Voices Bolder, Faster What we do: We are developing targeted therapies that are designed to address the complex and variable needs of people with autoimmune diseases.

Achieve all of the above with a simple, commercially attractive subcutaneous injection Demonstrate minimal to no impact on LDL Demonstrate minimal to no impact on albumin 4 Goals for the Phase 1 Program Demonstrate potential best-in-class IgG reductions similar to batoclimab Note: Based on data to date
5 Best-in-Class Potential for IMVT-1402 – Why it Matters FcRn inhibition is a proven mechanism with broad potential applicability based on targeted reduction of IgG as an improved and more targeted modality Evidence across broad range of auto-antibody conditions that deeper IgG reduction correlates with greater efficacy Immunovant has the potential to create a unique and class-leading portfolio of indications with IMVT-1402 IMVT-1402 demonstrates potentially best-in-class IgG reduction, similar to batoclimab, delivered via a simple subcutaneous injection and with minimal to no impact on albumin and LDL, similar to placebo

Multiple-Ascending Subcutaneous Doses (Once-weekly dosing x 4 weeks)

-100% -80% -60% -40% -20% 0% 20% 40% Day IMVT-1402: Multiple-ascending SC Dose IMVT-1402: MAD SC 600mg Group Mean IMVT-1402: MAD SC 300mg Group Mean IMVT-1402: MAD SC Placebo (600mg) Group Mean IMVT-1402: MAD SC Placebo (300mg) Group Mean -40% -30% -20% -10% 0% 10% 20% Day IMVT-1402: Multiple-ascending SC Dose IMVT-1402: MAD SC 600mg Group Mean IMVT-1402: MAD SC 300mg Group Mean IMVT-1402: MAD SC Placebo (600mg) Group Mean IMVT-1402: MAD SC Placebo (300mg) Group Mean -50% -40% -30% -20% -10% 0% 10% 20% 30% 40% 50% Day IMVT-1402: Multiple-ascending SC Dose IMVT-1402: MAD SC 600mg Group Mean IMVT-1402: MAD SC 300mg Group Mean IMVT-1402: MAD SC Placebo (600mg) Group Mean IMVT-1402: MAD SC Placebo (300mg) Group Mean IgG % change over time IMVT-1402 600mg MAD Data Consistent with 300mg MAD Data 7Dose administration Albumin % change over time LDL % change over time
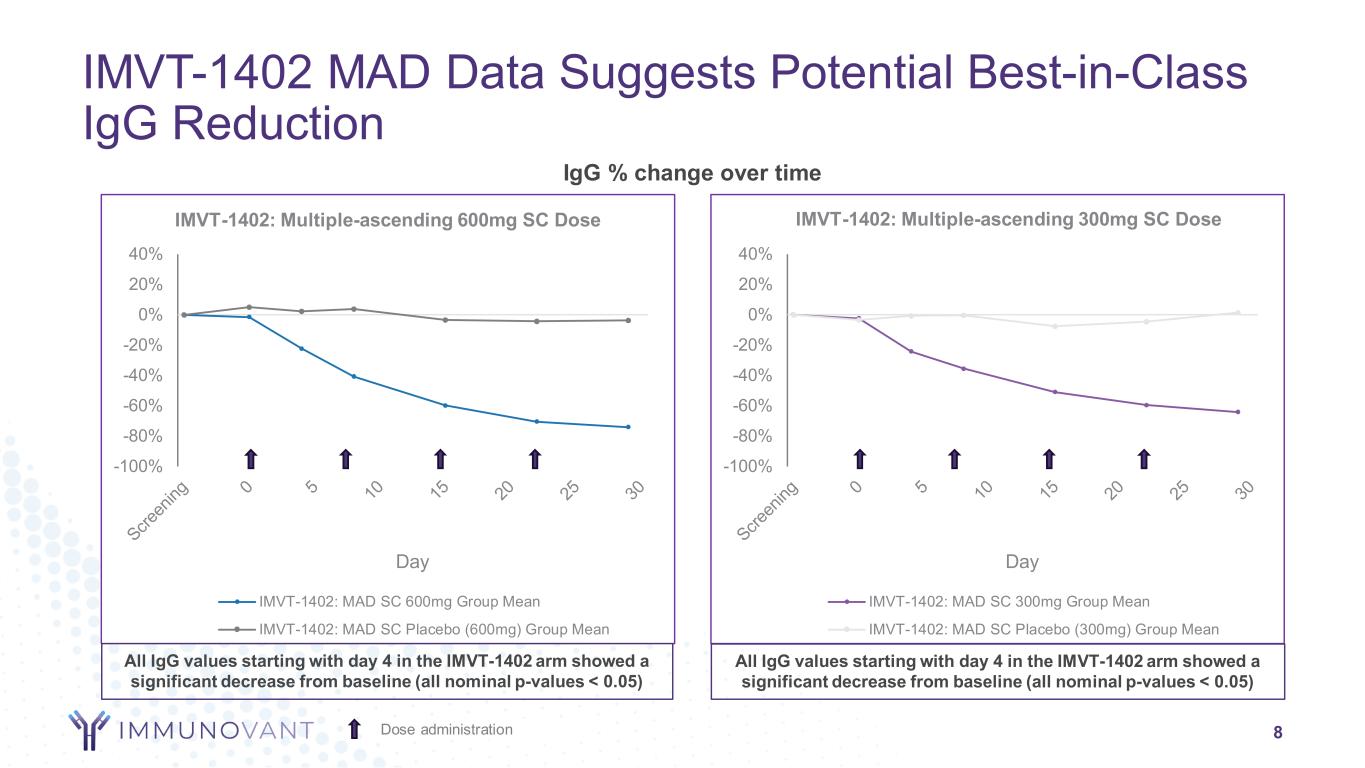
-100% -80% -60% -40% -20% 0% 20% 40% Day IMVT-1402: Multiple-ascending 600mg SC Dose IMVT-1402: MAD SC 600mg Group Mean IMVT-1402: MAD SC Placebo (600mg) Group Mean -100% -80% -60% -40% -20% 0% 20% 40% Day IMVT-1402: Multiple-ascending 300mg SC Dose IMVT-1402: MAD SC 300mg Group Mean IMVT-1402: MAD SC Placebo (300mg) Group Mean IgG % change over time IMVT-1402 MAD Data Suggests Potential Best-in-Class IgG Reduction 8Dose administration All IgG values starting with day 4 in the IMVT-1402 arm showed a significant decrease from baseline (all nominal p-values < 0.05) All IgG values starting with day 4 in the IMVT-1402 arm showed a significant decrease from baseline (all nominal p-values < 0.05)

-100% -80% -60% -40% -20% 0% 20% 40% 0 5 10 15 20 25 30 Day Multiple-ascending SC Dose IMVT-1402: MAD SC 600mg Group Mean IMVT-1402: MAD SC 300mg Group Mean IMVT-1402: MAD SC Placebo (600mg) Group Mean IMVT-1402: MAD SC Placebo (300mg) Group Mean Batoclimab: MAD SC 680mg Group Mean Batoclimab: MAD SC 340mg Group Mean Batoclimab: MAD SC Placebo (680mg) Group Mean Batoclimab: MAD SC Placebo (340mg) Group Mean IMVT-1402 MAD Data Suggests Potential Best-in-Class IgG Reduction Similar to Batoclimab* 9Dose administration *Data presented are from separate clinical trials, not a head-to-head study, conducted by Immunovant IgG % change over time
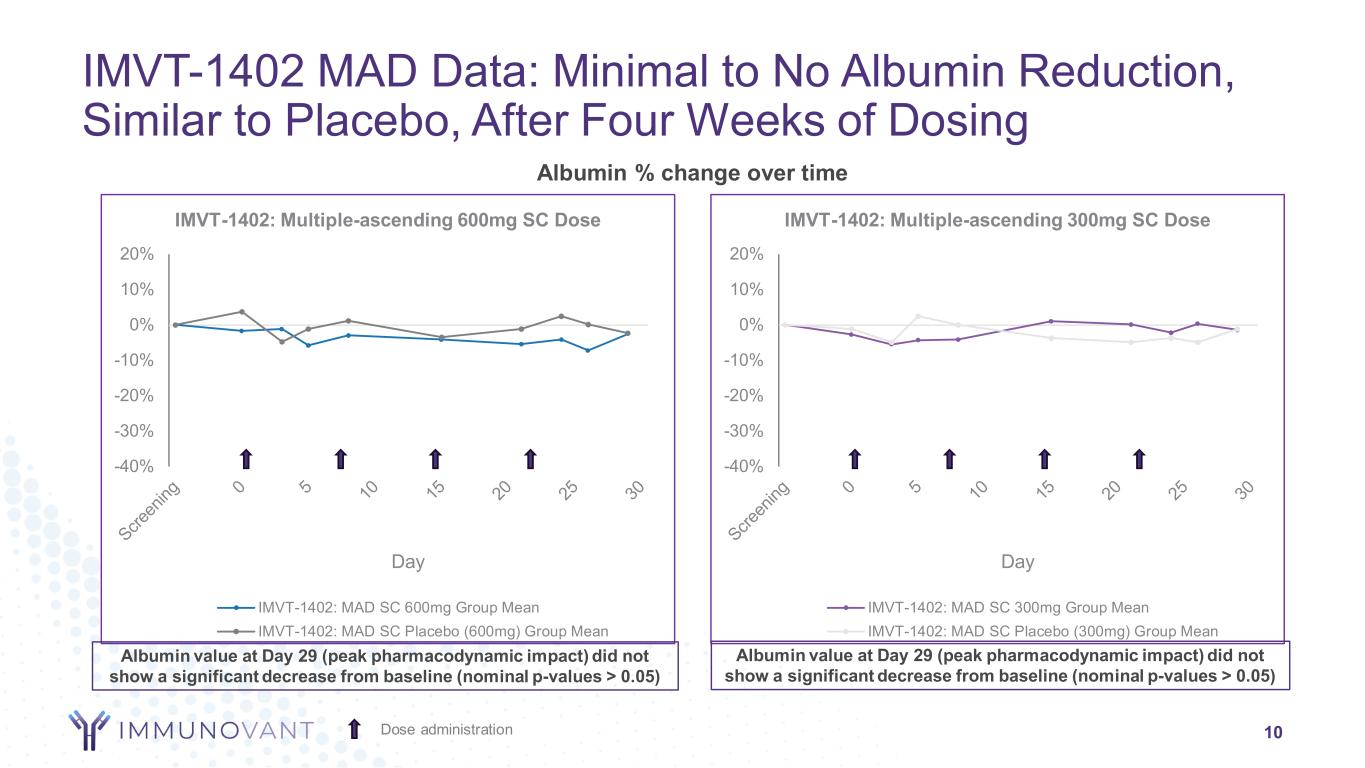
-40% -30% -20% -10% 0% 10% 20% Day IMVT-1402: Multiple-ascending 300mg SC Dose IMVT-1402: MAD SC 300mg Group Mean IMVT-1402: MAD SC Placebo (300mg) Group Mean -40% -30% -20% -10% 0% 10% 20% Day IMVT-1402: Multiple-ascending 600mg SC Dose IMVT-1402: MAD SC 600mg Group Mean IMVT-1402: MAD SC Placebo (600mg) Group Mean Albumin % change over time IMVT-1402 MAD Data: Minimal to No Albumin Reduction, Similar to Placebo, After Four Weeks of Dosing 10Dose administration Albumin value at Day 29 (peak pharmacodynamic impact) did not show a significant decrease from baseline (nominal p-values > 0.05) Albumin value at Day 29 (peak pharmacodynamic impact) did not show a significant decrease from baseline (nominal p-values > 0.05)
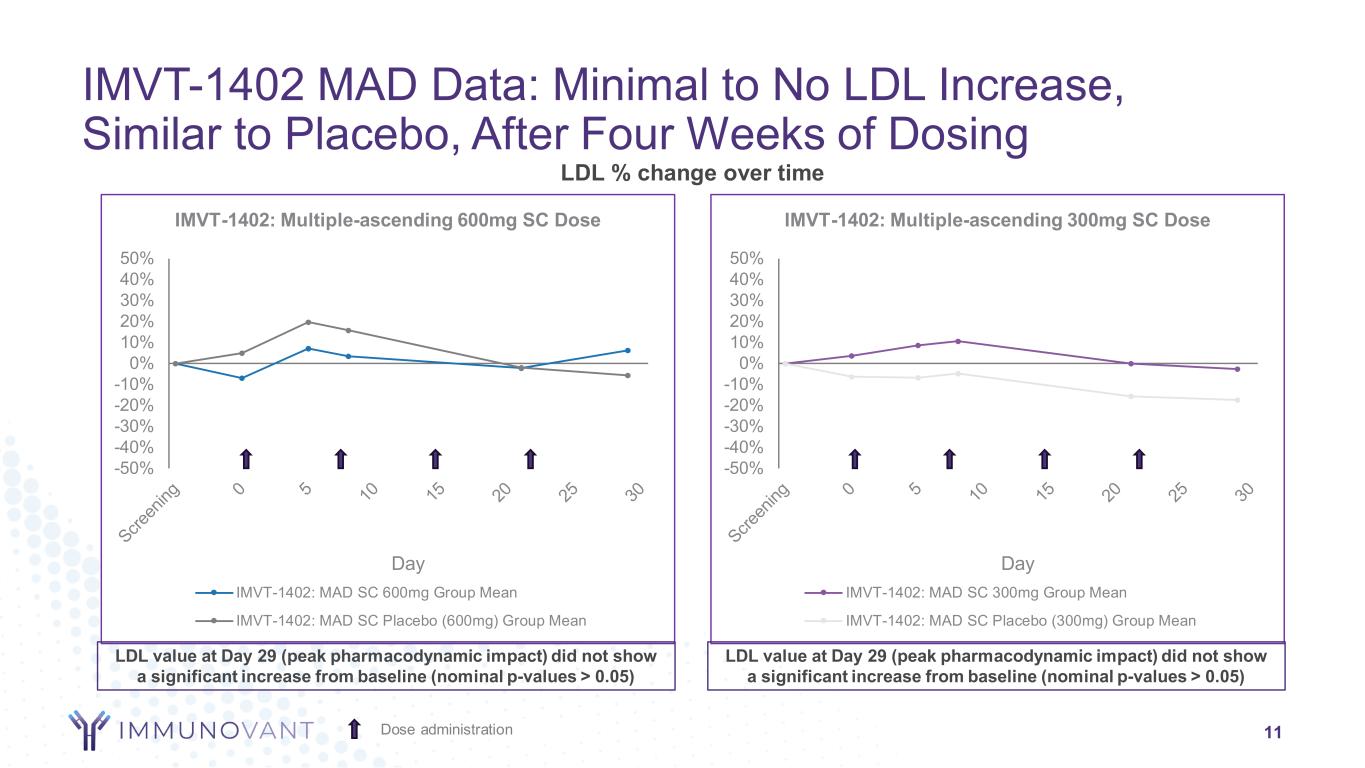
-50% -40% -30% -20% -10% 0% 10% 20% 30% 40% 50% Day IMVT-1402: Multiple-ascending 600mg SC Dose IMVT-1402: MAD SC 600mg Group Mean IMVT-1402: MAD SC Placebo (600mg) Group Mean -50% -40% -30% -20% -10% 0% 10% 20% 30% 40% 50% Day IMVT-1402: Multiple-ascending 300mg SC Dose IMVT-1402: MAD SC 300mg Group Mean IMVT-1402: MAD SC Placebo (300mg) Group Mean LDL % change over time IMVT-1402 MAD Data: Minimal to No LDL Increase, Similar to Placebo, After Four Weeks of Dosing 11Dose administration LDL value at Day 29 (peak pharmacodynamic impact) did not show a significant increase from baseline (nominal p-values > 0.05) LDL value at Day 29 (peak pharmacodynamic impact) did not show a significant increase from baseline (nominal p-values > 0.05)
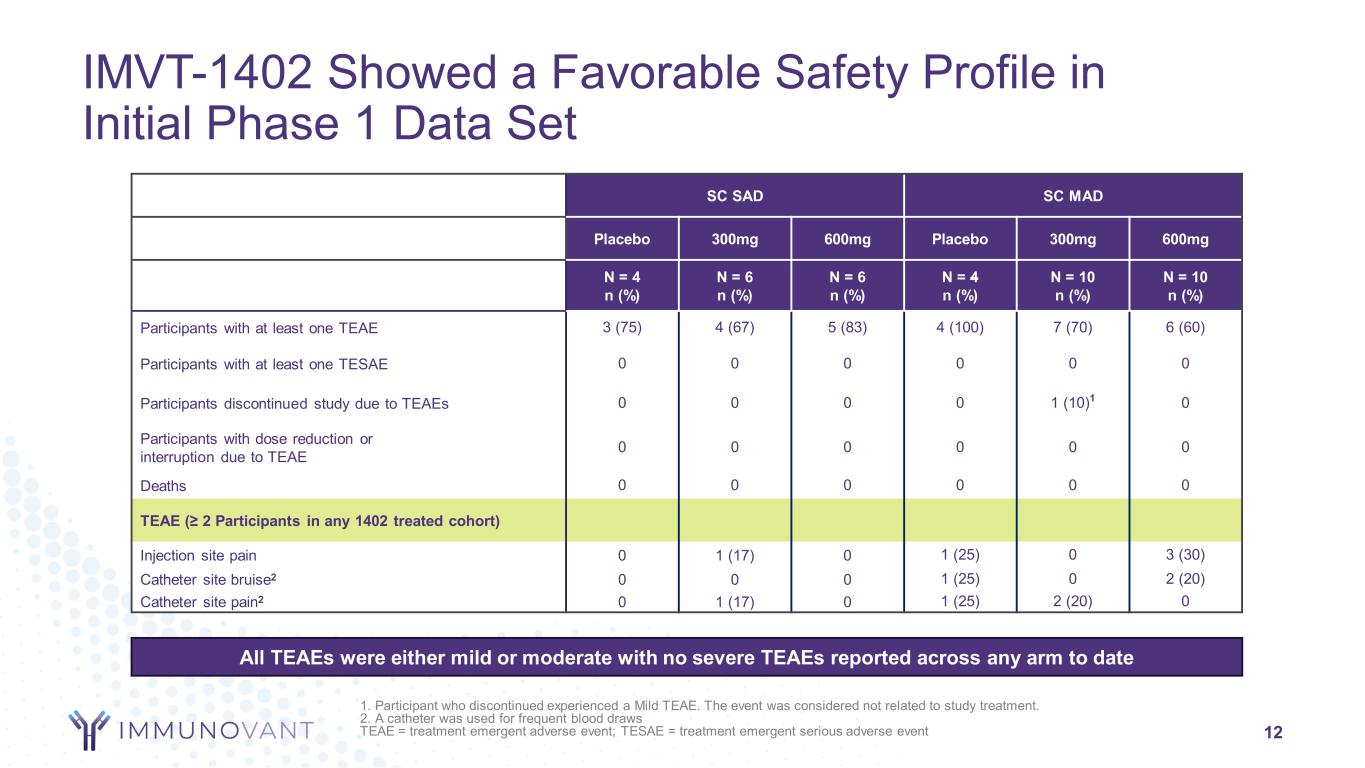
12 SC SAD SC MAD Placebo 300mg 600mg Placebo 300mg 600mg N = 4 n (%) N = 6 n (%) N = 6 n (%) N = 4 n (%) N = 10 n (%) N = 10 n (%) Participants with at least one TEAE 3 (75) 4 (67) 5 (83) 4 (100) 7 (70) 6 (60) Participants with at least one TESAE 0 0 0 0 0 0 Participants discontinued study due to TEAEs 0 0 0 0 1 (10)1 0 Participants with dose reduction or interruption due to TEAE 0 0 0 0 0 0 Deaths 0 0 0 0 0 0 TEAE (≥ 2 Participants in any 1402 treated cohort) Injection site pain 0 1 (17) 0 1 (25) 0 3 (30) Catheter site bruise2 0 0 0 1 (25) 0 2 (20) Catheter site pain2 0 1 (17) 0 1 (25) 2 (20) 0 IMVT-1402 Showed a Favorable Safety Profile in Initial Phase 1 Data Set All TEAEs were either mild or moderate with no severe TEAEs reported across any arm to date 1. Participant who discontinued experienced a Mild TEAE. The event was considered not related to study treatment. 2. A catheter was used for frequent blood draws TEAE = treatment emergent adverse event; TESAE = treatment emergent serious adverse event

IgG Reduction and Clinical Efficacy Correlation
14 Best-in-Class Potential for IMVT-1402 – Why it Matters FcRn inhibition is a proven mechanism with broad potential applicability based on targeted reduction of IgG as an improved and more targeted modality Evidence across broad range of auto-antibody conditions that deeper IgG reduction correlates with greater efficacy Immunovant has the potential to create a unique and class-leading portfolio of indications with IMVT-1402 IMVT-1402 demonstrates potentially best-in-class IgG reduction, similar to batoclimab, delivered via a simple subcutaneous injection and with minimal to no impact on albumin and LDL, similar to placebo
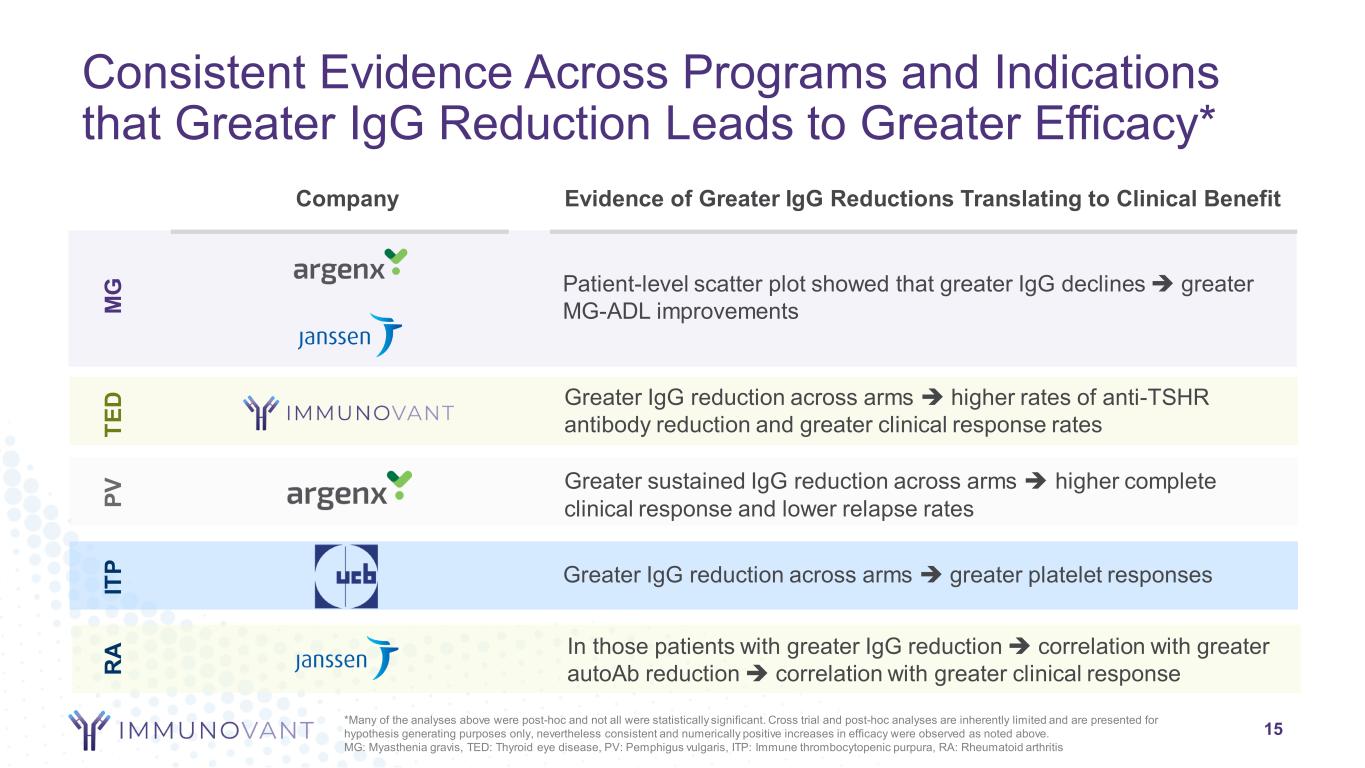
Consistent Evidence Across Programs and Indications that Greater IgG Reduction Leads to Greater Efficacy* Company Evidence of Greater IgG Reductions Translating to Clinical Benefit M G TE D IT P Patient-level scatter plot showed that greater IgG declines greater MG-ADL improvements Greater IgG reduction across arms higher rates of anti-TSHR antibody reduction and greater clinical response rates Greater IgG reduction across arms greater platelet responses PV Greater sustained IgG reduction across arms higher complete clinical response and lower relapse rates 15 R A In those patients with greater IgG reduction correlation with greater autoAb reduction correlation with greater clinical response *Many of the analyses above were post-hoc and not all were statistically significant. Cross trial and post-hoc analyses are inherently limited and are presented for hypothesis generating purposes only, nevertheless consistent and numerically positive increases in efficacy were observed as noted above. MG: Myasthenia gravis, TED: Thyroid eye disease, PV: Pemphigus vulgaris, ITP: Immune thrombocytopenic purpura, RA: Rheumatoid arthritis
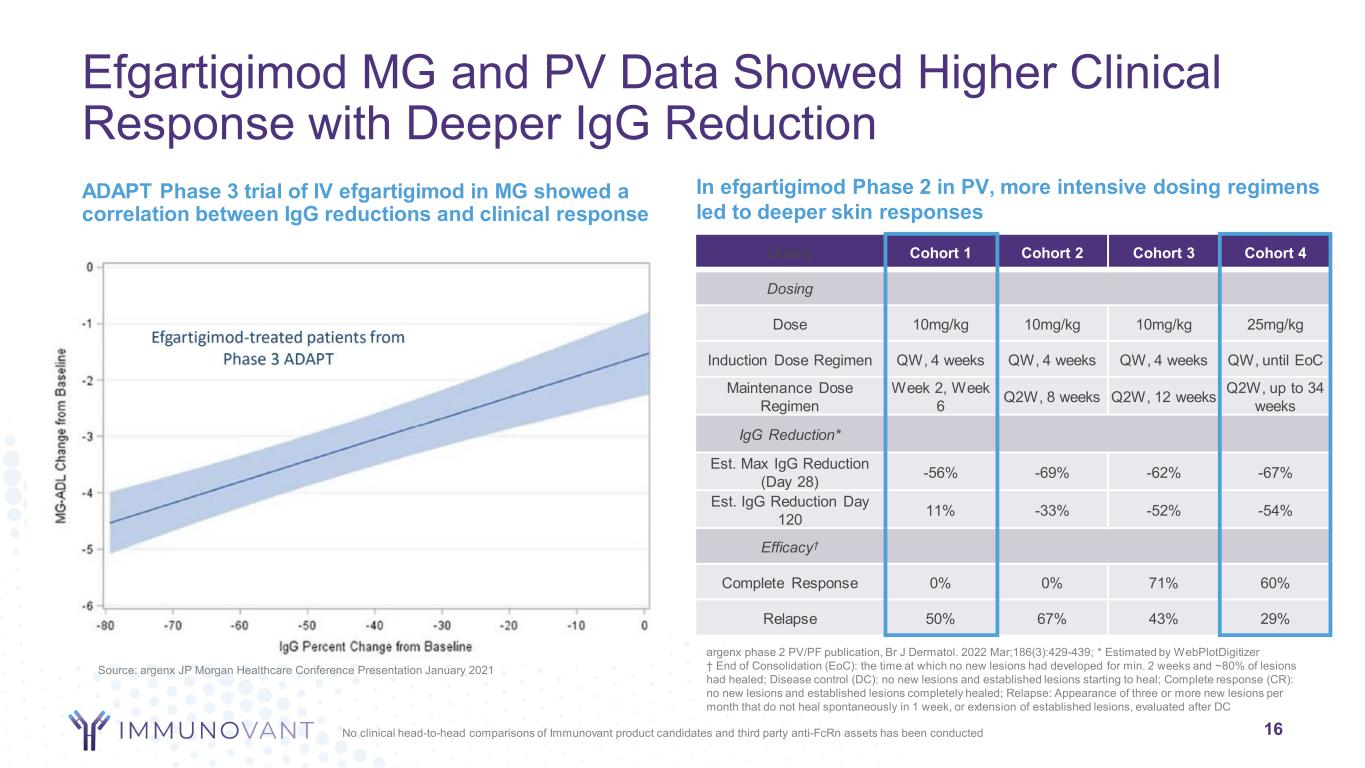
Efgartigimod MG and PV Data Showed Higher Clinical Response with Deeper IgG Reduction ADAPT Phase 3 trial of IV efgartigimod in MG showed a correlation between IgG reductions and clinical response 16 argenx phase 2 PV/PF publication, Br J Dermatol. 2022 Mar;186(3):429-439; * Estimated by WebPlotDigitizer † End of Consolidation (EoC): the time at which no new lesions had developed for min. 2 weeks and ~80% of lesions had healed; Disease control (DC): no new lesions and established lesions starting to heal; Complete response (CR): no new lesions and established lesions completely healed; Relapse: Appearance of three or more new lesions per month that do not heal spontaneously in 1 week, or extension of established lesions, evaluated after DC In efgartigimod Phase 2 in PV, more intensive dosing regimens led to deeper skin responses Source: argenx JP Morgan Healthcare Conference Presentation January 2021 Dosing Cohort 1 Cohort 2 Cohort 3 Cohort 4 Dosing Dose 10mg/kg 10mg/kg 10mg/kg 25mg/kg Induction Dose Regimen QW, 4 weeks QW, 4 weeks QW, 4 weeks QW, until EoC Maintenance Dose Regimen Week 2, Week 6 Q2W, 8 weeks Q2W, 12 weeks Q2W, up to 34 weeks IgG Reduction* Est. Max IgG Reduction (Day 28) -56% -69% -62% -67% Est. IgG Reduction Day 120 11% -33% -52% -54% Efficacy† Complete Response 0% 0% 71% 60% Relapse 50% 67% 43% 29% No clinical head-to-head comparisons of Immunovant product candidates and third party anti-FcRn assets has been conducted

Nipocalimab MG and RA Data Showed Higher Clinical Response with Deeper IgG Reduction Nipocalimab Phase 2 trial in MG showed a correlation between IgG reductions and clinical response 17 Nipocalimab Phase 2 trial in RA showed a correlation between auto-Ab reductions and clinical response Source: Pharmacodynamic effects of nipocalimab in patients with moderate to severe active rheumatoid arthritis (RA): Results from the multicenter, randomized, double-blinded, placebo-controlled Phase 2A IRIS-RA study. Janssen Research & Development, ACR poster, November 2023. No clinical head-to-head comparisons of Immunovant product candidates and third party anti-FcRn assets has been conducted Source: Momenta Vivacity-MG Interim Phase 2 Investor Presentation, 2020
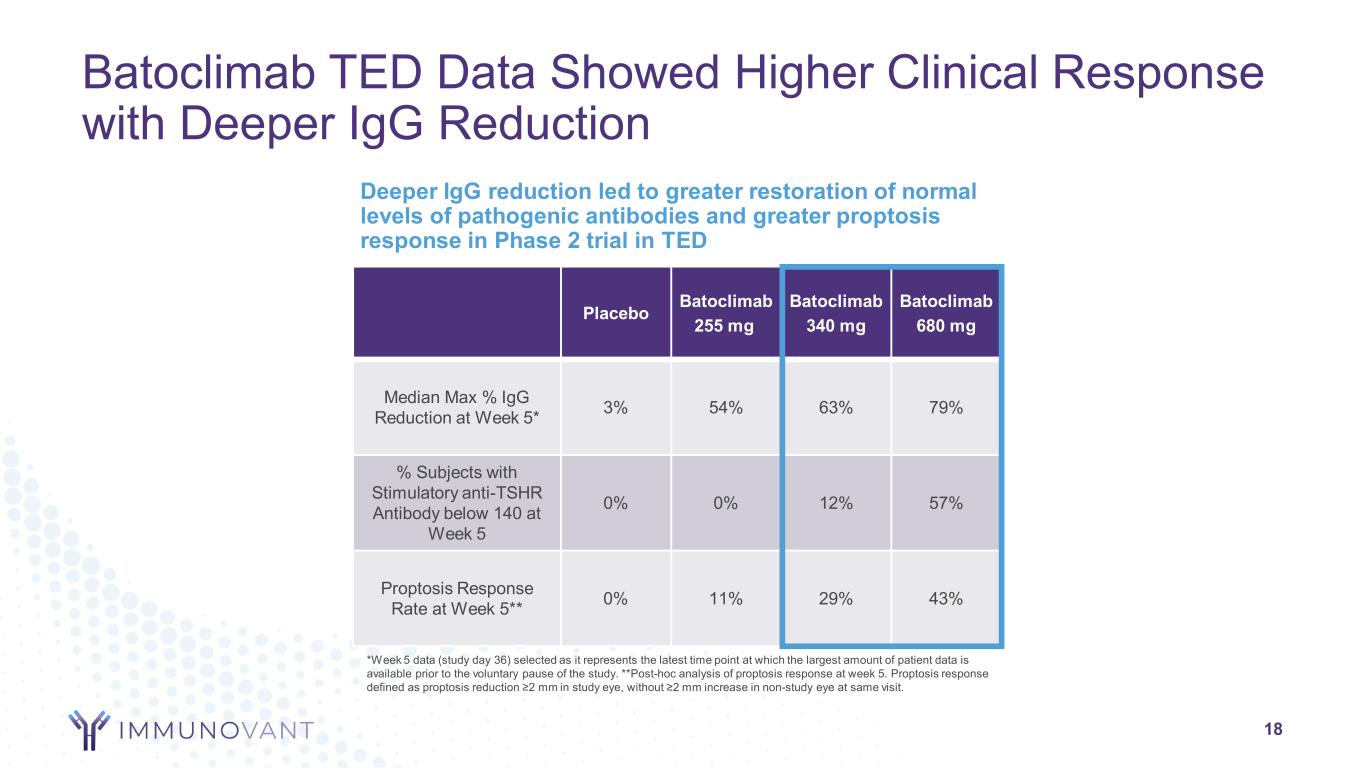
Batoclimab TED Data Showed Higher Clinical Response with Deeper IgG Reduction 18 Deeper IgG reduction led to greater restoration of normal levels of pathogenic antibodies and greater proptosis response in Phase 2 trial in TED Placebo Batoclimab 255 mg Batoclimab 340 mg Batoclimab 680 mg Median Max % IgG Reduction at Week 5* 3% 54% 63% 79% % Subjects with Stimulatory anti-TSHR Antibody below 140 at Week 5 0% 0% 12% 57% Proptosis Response Rate at Week 5** 0% 11% 29% 43% *Week 5 data (study day 36) selected as it represents the latest time point at which the largest amount of patient data is available prior to the voluntary pause of the study. **Post-hoc analysis of proptosis response at week 5. Proptosis response defined as proptosis reduction ≥2 mm in study eye, without ≥2 mm increase in non-study eye at same visit.
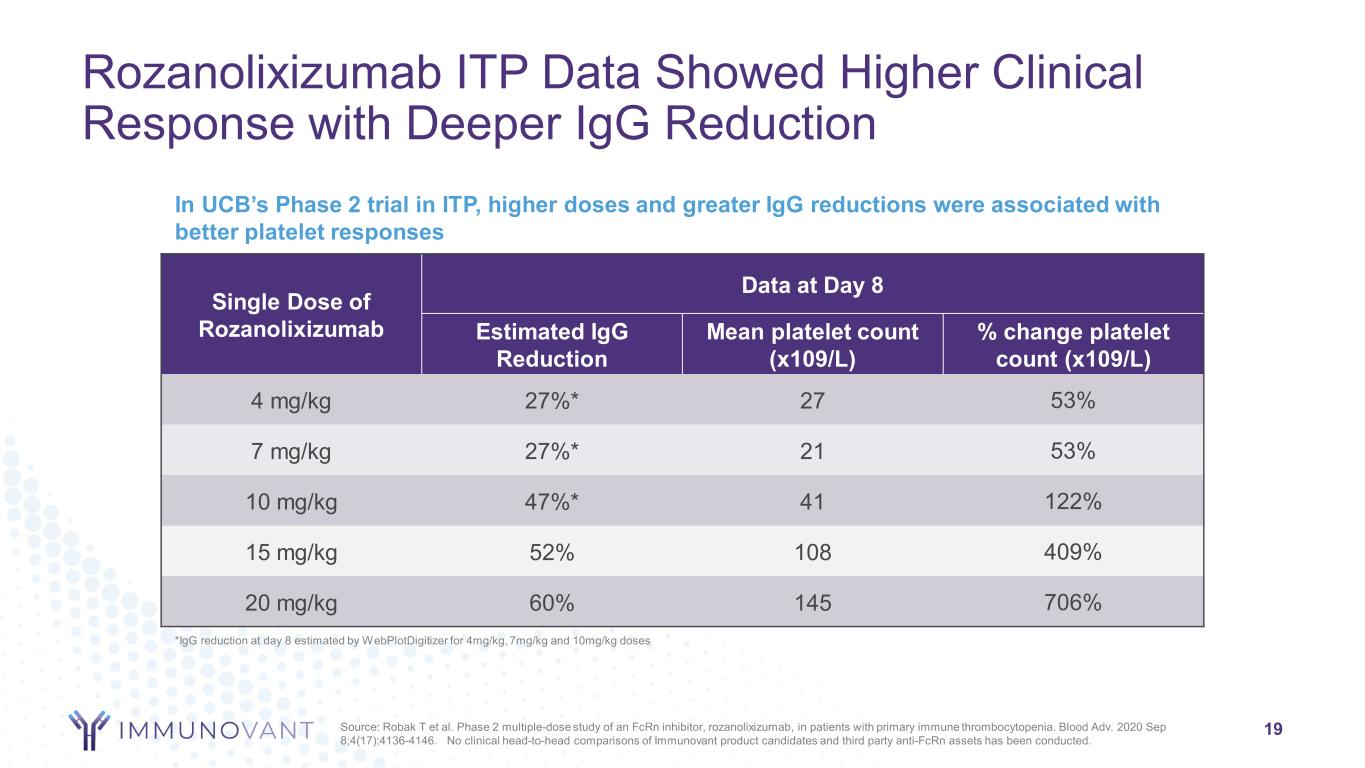
Rozanolixizumab ITP Data Showed Higher Clinical Response with Deeper IgG Reduction 19 In UCB’s Phase 2 trial in ITP, higher doses and greater IgG reductions were associated with better platelet responses Single Dose of Rozanolixizumab Data at Day 8 Estimated IgG Reduction Mean platelet count (x109/L) % change platelet count (x109/L) 4 mg/kg 27%* 27 53% 7 mg/kg 27%* 21 53% 10 mg/kg 47%* 41 122% 15 mg/kg 52% 108 409% 20 mg/kg 60% 145 706% *IgG reduction at day 8 estimated by WebPlotDigitizer for 4mg/kg, 7mg/kg and 10mg/kg doses Source: Robak T et al. Phase 2 multiple-dose study of an FcRn inhibitor, rozanolixizumab, in patients with primary immune thrombocytopenia. Blood Adv. 2020 Sep 8;4(17):4136-4146. No clinical head-to-head comparisons of Immunovant product candidates and third party anti-FcRn assets has been conducted.

Portfolio Development for IMVT-1402
21 Best-in-Class Potential for IMVT-1402 – Why it Matters FcRn inhibition is a proven mechanism with broad potential applicability based on targeted reduction of IgG as an improved and more targeted modality Evidence across broad range of auto-antibody conditions that deeper IgG reduction correlates with greater efficacy Immunovant has the potential to create a unique and class-leading portfolio of indications with IMVT-1402 IMVT-1402 demonstrates potentially best-in-class IgG reduction, similar to batoclimab, delivered via a simple subcutaneous injection and with minimal to no impact on albumin and LDL, similar to placebo
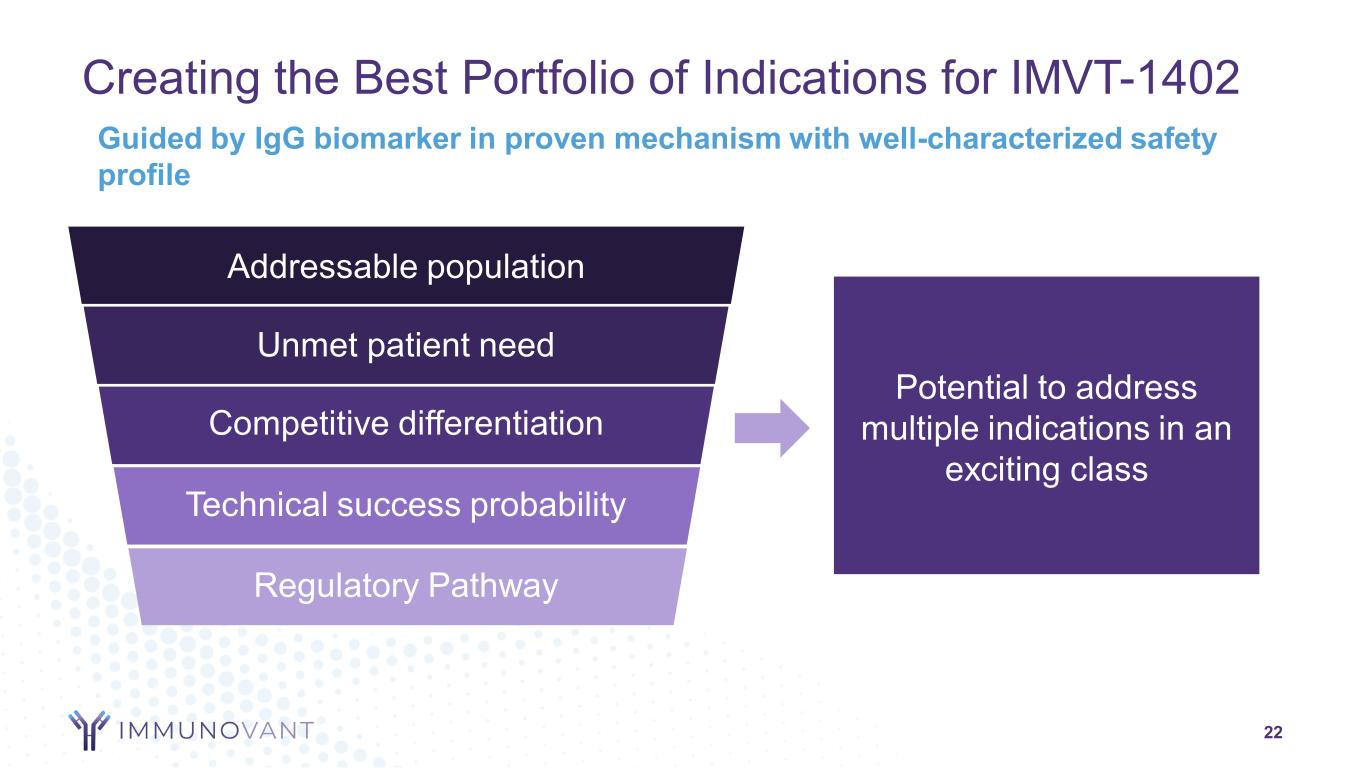
Creating the Best Portfolio of Indications for IMVT-1402 22 Potential to address multiple indications in an exciting class Guided by IgG biomarker in proven mechanism with well-characterized safety profile Addressable population Unmet patient need Competitive differentiation Technical success probability Regulatory Pathway
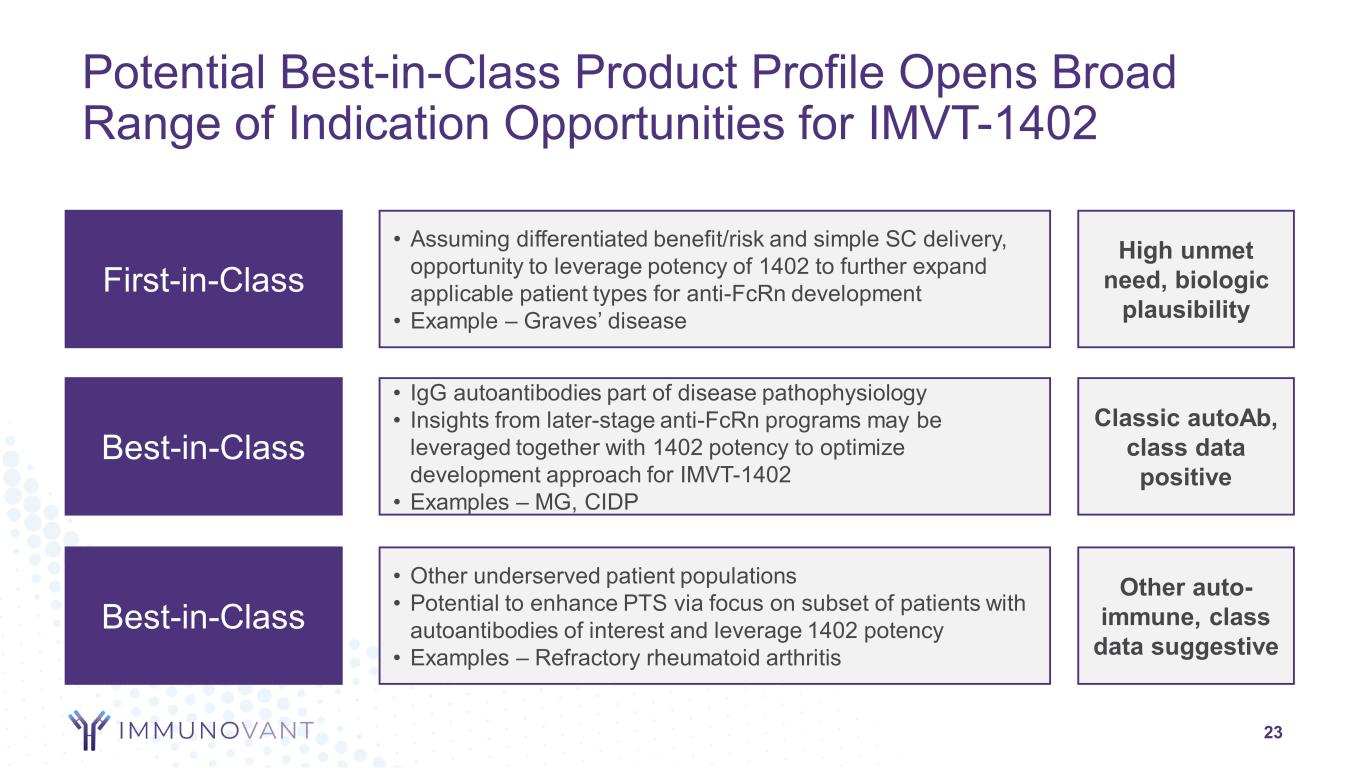
Potential Best-in-Class Product Profile Opens Broad Range of Indication Opportunities for IMVT-1402 23 Best-in-Class • Other underserved patient populations • Potential to enhance PTS via focus on subset of patients with autoantibodies of interest and leverage 1402 potency • Examples – Refractory rheumatoid arthritis Other auto- immune, class data suggestive First-in-Class • Assuming differentiated benefit/risk and simple SC delivery, opportunity to leverage potency of 1402 to further expand applicable patient types for anti-FcRn development • Example – Graves’ disease High unmet need, biologic plausibility Best-in-Class • IgG autoantibodies part of disease pathophysiology • Insights from later-stage anti-FcRn programs may be leveraged together with 1402 potency to optimize development approach for IMVT-1402 • Examples – MG, CIDP Classic autoAb, class data positive
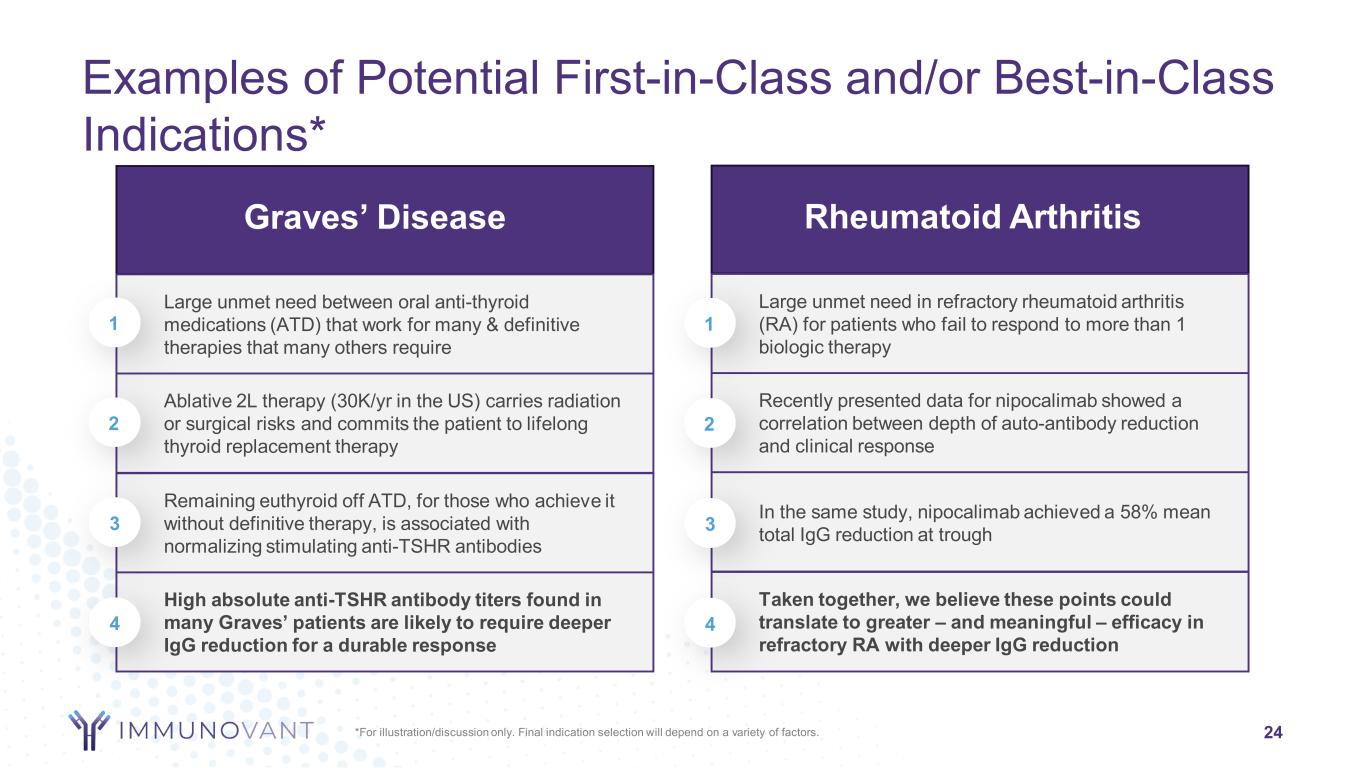
Graves’ Disease Large unmet need between oral anti-thyroid medications (ATD) that work for many & definitive therapies that many others require Ablative 2L therapy (30K/yr in the US) carries radiation or surgical risks and commits the patient to lifelong thyroid replacement therapy Remaining euthyroid off ATD, for those who achieve it without definitive therapy, is associated with normalizing stimulating anti-TSHR antibodies High absolute anti-TSHR antibody titers found in many Graves’ patients are likely to require deeper IgG reduction for a durable response Rheumatoid Arthritis Large unmet need in refractory rheumatoid arthritis (RA) for patients who fail to respond to more than 1 biologic therapy Recently presented data for nipocalimab showed a correlation between depth of auto-antibody reduction and clinical response In the same study, nipocalimab achieved a 58% mean total IgG reduction at trough Taken together, we believe these points could translate to greater – and meaningful – efficacy in refractory RA with deeper IgG reduction Examples of Potential First-in-Class and/or Best-in-Class Indications* 24*For illustration/discussion only. Final indication selection will depend on a variety of factors. 1 2 3 4 1 2 3 4

Concluding Thoughts
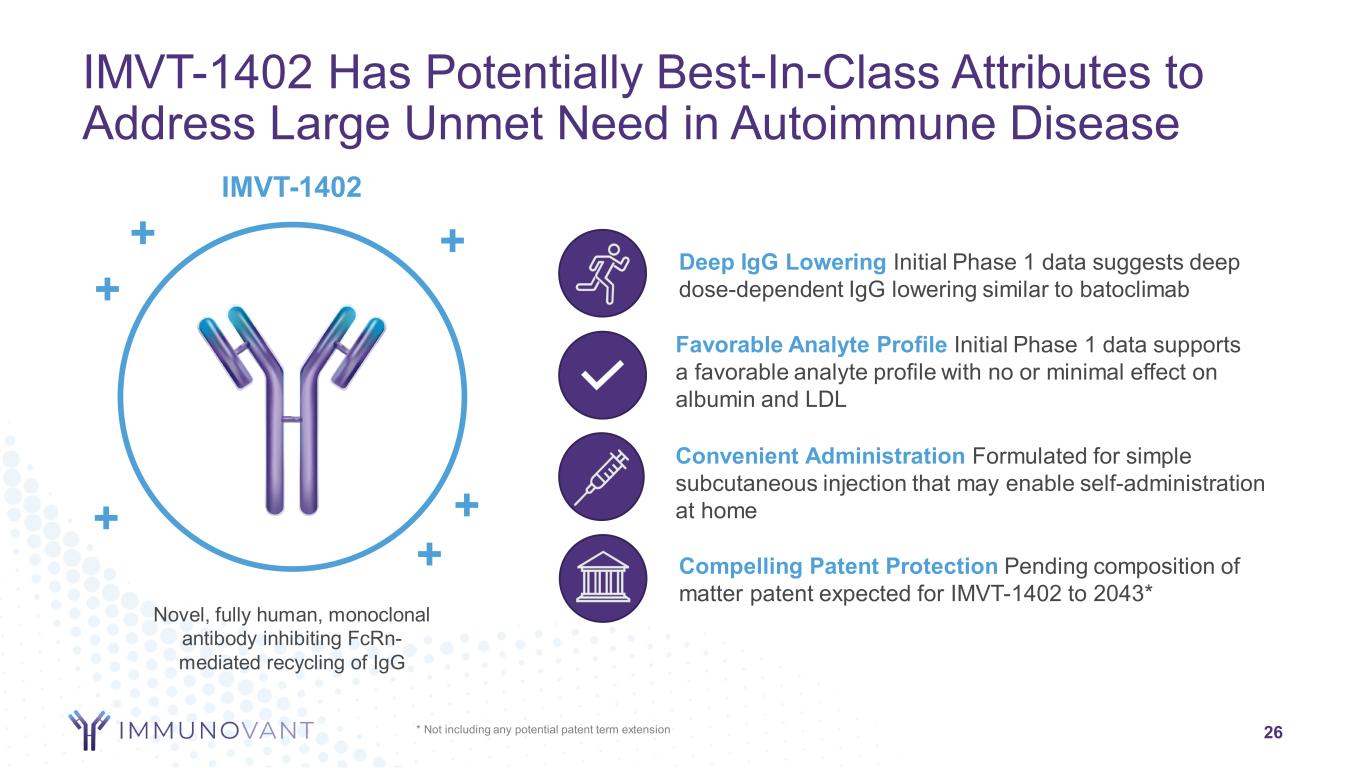
Favorable Analyte Profile Initial Phase 1 data supports a favorable analyte profile with no or minimal effect on albumin and LDL * Not including any potential patent term extension 26 IMVT-1402 Has Potentially Best-In-Class Attributes to Address Large Unmet Need in Autoimmune Disease Novel, fully human, monoclonal antibody inhibiting FcRn- mediated recycling of IgG + IMVT-1402 ++ + + + Convenient Administration Formulated for simple subcutaneous injection that may enable self-administration at home Compelling Patent Protection Pending composition of matter patent expected for IMVT-1402 to 2043* Deep IgG Lowering Initial Phase 1 data suggests deep dose-dependent IgG lowering similar to batoclimab
Concluding Thoughts 27 The proven anti- FcRn class keeps getting more exciting IMIVT-1402’s profile and potency creates an exciting range of potential indications 2024 will be a big year for IMVT-1402 as we announce a broad portfolio of pivotal trials and POCs