
Corporate Presentation January 2023 Exhibit 99.1

Forward-looking Statements 2 This presentation contains forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. The use of words such as "can," “may,” “might,” “will,” “would,” “should,” “expect,” “believe,” “estimate,” “design,” “plan,” "intend," and other similar expressions are intended to identify forward-looking statements. Such forward looking statements include Immunovant’s expectations regarding patient enrollment, timing, design, and results of clinical trials of its product candidates and indication selections; Immunovant's plan to develop batoclimab and IMVT-1402 across a broad range of autoimmune indications; expectations with respect to the safety and monitoring plan and size of the safety database for these planned clinical trials; the timing of discussions with regulatory agencies; the size and growth of the potential markets for Immunovant's product candidates and indication selections; Immunovant’s plan to explore in subsequent study periods follow-on treatment with alternative dosing regimens; Immunovant's expectations regarding its cash runway; Immunovant's beliefs regarding the potential benefits of batoclimab’s and IMVT-1402's unique product attributes; and Immunovant's expectations regarding the issuance and term of any pending patents. All forward-looking statements are based on estimates and assumptions by Immunovant’s management that, although Immunovant believes to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that Immunovant expected. Such risks and uncertainties include, among others: initial results or other preliminary analyses or results of early clinical trials may not be predictive of final trial results or of the results of later clinical trials; results of animal studies may not be predictive of results in humans; the timing and availability of data from clinical trials; the timing of discussions with regulatory agencies, as well as regulatory submissions and potential approvals; the continued development of Immunovant’s product candidates, including the timing of the commencement of additional clinical trials and resumption of current trials; Immunovant’s scientific approach, clinical trial design, indication selection, and general development progress; future clinical trials may not confirm any safety, potency, or other product characteristics described or assumed in this presentation; any product candidate that Immunovant develops may not progress through clinical development or receive required regulatory approvals within expected timelines or at all; Immunovant's pending composition of matter patent for IMVT-1402 may not be issued; Immunovant’s product candidates may not be beneficial to patients, or even if approved by regulatory authorities, successfully commercialized; the potential impact of the ongoing COVID-19 pandemic, geopolitical tensions, and adverse macroeconomic conditions on Immunovant’s clinical development plans and timelines; Immunovant’s business is heavily dependent on the successful development, regulatory approval and commercialization of batoclimab and IMVT-1402; Immunovant is at an early stage in development for IMVT-1402 and in various stages of clinical development for batoclimab; and Immunovant will require additional capital to fund its operations and advance batoclimab and IMVT-1402 through clinical development. These and other risks and uncertainties are more fully described in Immunovant’s periodic and other reports filed with the Securities and Exchange Commission (SEC), including in the section titled “Risk Factors” in Immunovant’s most recent Annual Report on Form 10-K, its Form 10-Q filed with the SEC on November 4, 2022, and Immunovant’s subsequent filings with the SEC. Any forward-looking statement speaks only as of the date on which it was made. Immunovant undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. All trademarks, trade names, service marks, and copyrights appearing in this presentation are the property of their respective owners. Dates used in this presentation refer to the applicable calendar year unless otherwise noted.
Our Vision: Normal Lives for People with Autoimmune Disease Core Values 3 Love Trailblazing All Voices Bolder, Faster
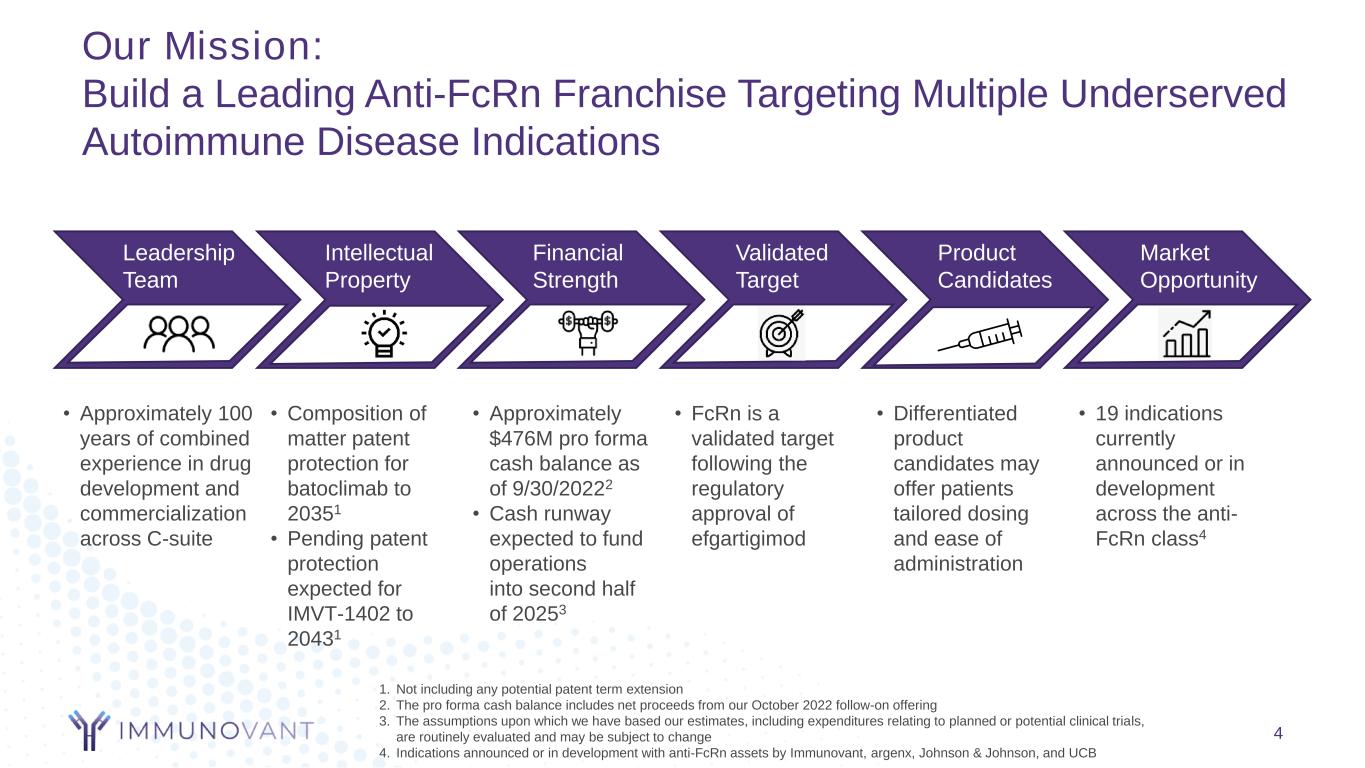
Our Mission: Build a Leading Anti-FcRn Franchise Targeting Multiple Underserved Autoimmune Disease Indications 4 Leadership Team Intellectual Property Financial Strength Validated Target Product Candidates Market Opportunity • Approximately 100 years of combined experience in drug development and commercialization across C-suite • Approximately $476M pro forma cash balance as of 9/30/20222 • Cash runway expected to fund operations into second half of 20253 • FcRn is a validated target following the regulatory approval of efgartigimod • Differentiated product candidates may offer patients tailored dosing and ease of administration • 19 indications currently announced or in development across the anti- FcRn class4 • Composition of matter patent protection for batoclimab to 20351 • Pending patent protection expected for IMVT-1402 to 20431 1. Not including any potential patent term extension 2. The pro forma cash balance includes net proceeds from our October 2022 follow-on offering 3. The assumptions upon which we have based our estimates, including expenditures relating to planned or potential clinical trials, are routinely evaluated and may be subject to change 4. Indications announced or in development with anti-FcRn assets by Immunovant, argenx, Johnson & Johnson, and UCB

Our Leadership Team: A Tight-knit Group of Experienced Executives 5 Peter Salzmann, MD MBA Chief Executive Officer William L. Macias, MD PhD Chief Medical Officer Eva Renee Barnett, MBA Chief Financial Officer Julia G. Butchko, PhD Chief Development and Technology Officer Mark S. Levine Chief Legal Officer and Corporate Secretary
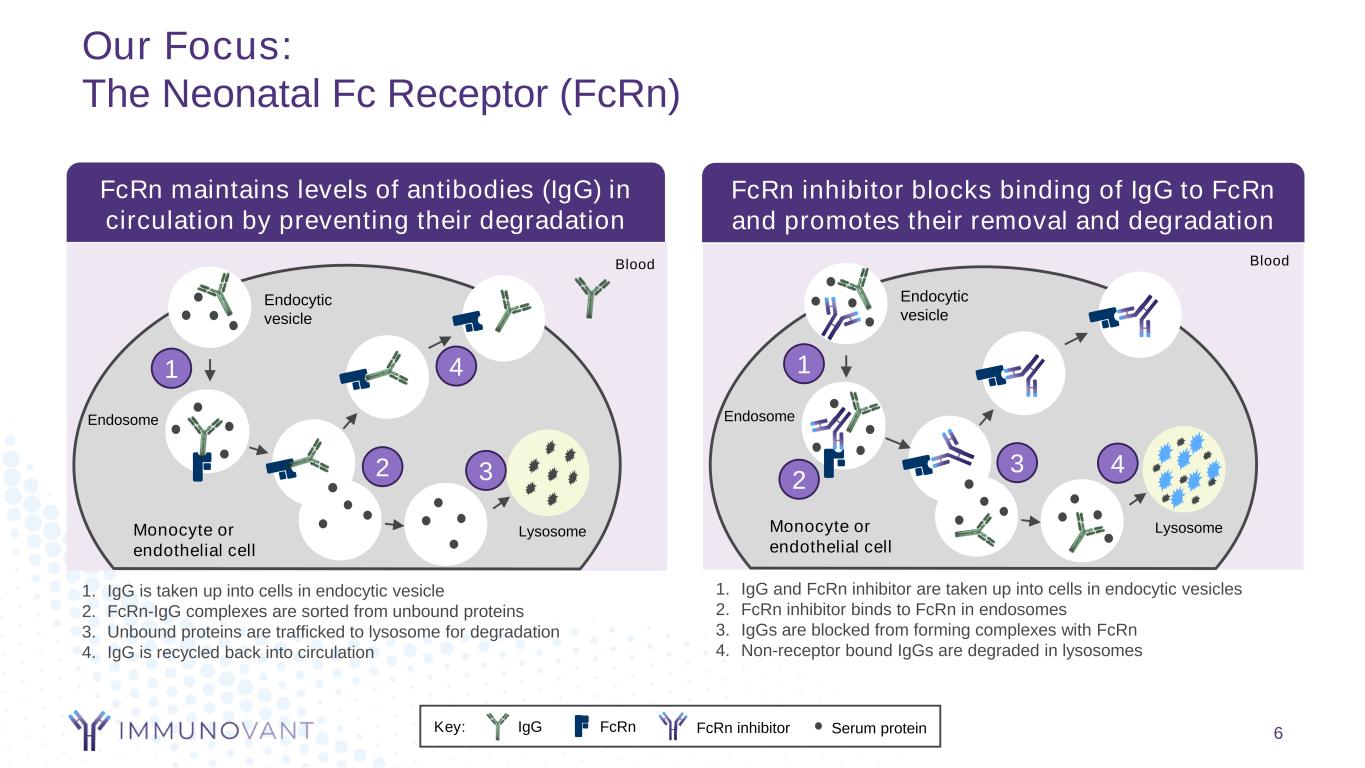
Our Focus: The Neonatal Fc Receptor (FcRn) 6 Blood Endocytic vesicle Endosome LysosomeMonocyte or endothelial cell 1 2 3 4 Key: Serum proteinIgG FcRn FcRn inhibitor Blood Endocytic vesicle Endosome LysosomeMonocyte or endothelial cell 1 2 3 4 FcRn maintains levels of antibodies (IgG) in circulation by preventing their degradation FcRn inhibitor blocks binding of IgG to FcRn and promotes their removal and degradation 1. IgG and FcRn inhibitor are taken up into cells in endocytic vesicles 2. FcRn inhibitor binds to FcRn in endosomes 3. IgGs are blocked from forming complexes with FcRn 4. Non-receptor bound IgGs are degraded in lysosomes 1. IgG is taken up into cells in endocytic vesicle 2. FcRn-IgG complexes are sorted from unbound proteins 3. Unbound proteins are trafficked to lysosome for degradation 4. IgG is recycled back into circulation
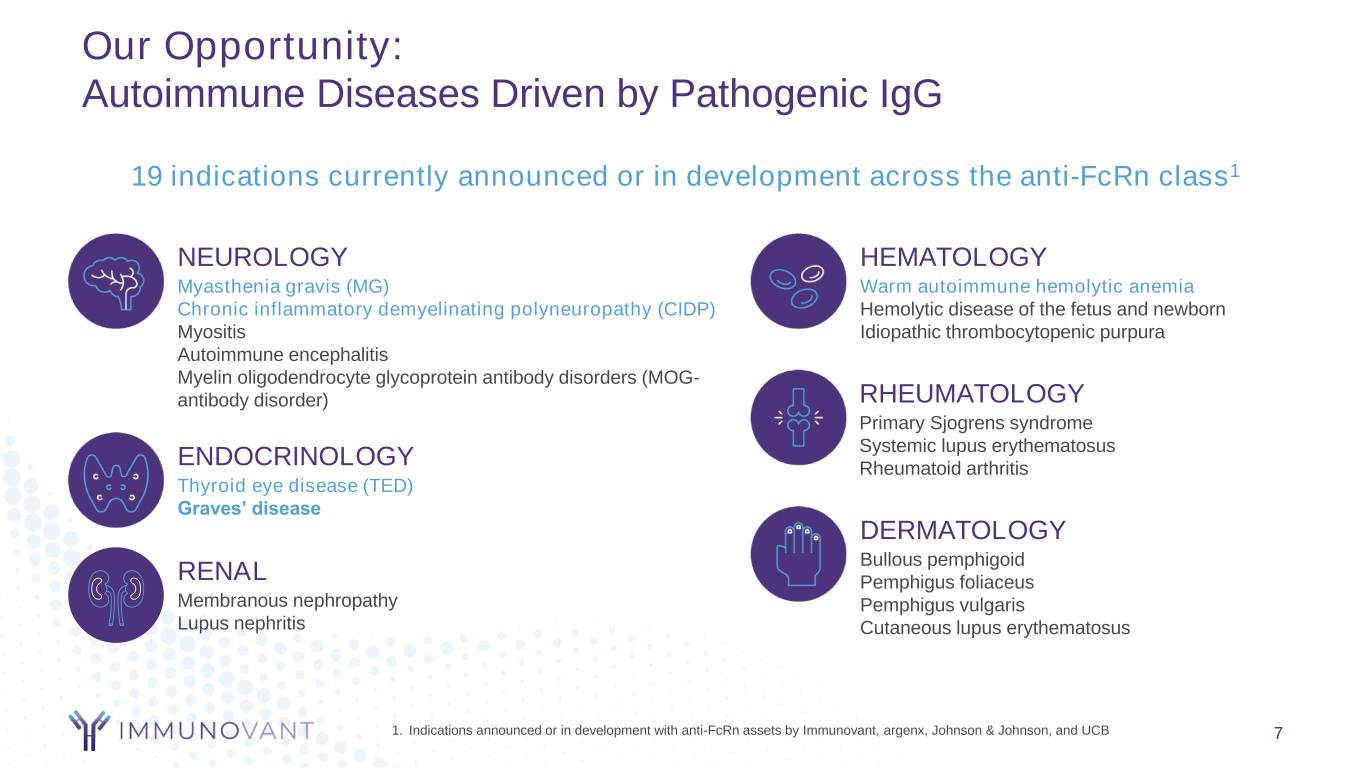
Our Opportunity: Autoimmune Diseases Driven by Pathogenic IgG 7 ENDOCRINOLOGY Thyroid eye disease (TED) Graves’ disease RHEUMATOLOGY Primary Sjogrens syndrome Systemic lupus erythematosus Rheumatoid arthritis NEUROLOGY Myasthenia gravis (MG) Chronic inflammatory demyelinating polyneuropathy (CIDP) Myositis Autoimmune encephalitis Myelin oligodendrocyte glycoprotein antibody disorders (MOG- antibody disorder) HEMATOLOGY Warm autoimmune hemolytic anemia Hemolytic disease of the fetus and newborn Idiopathic thrombocytopenic purpura DERMATOLOGY Bullous pemphigoid Pemphigus foliaceus Pemphigus vulgaris Cutaneous lupus erythematosus RENAL Membranous nephropathy Lupus nephritis 1. Indications announced or in development with anti-FcRn assets by Immunovant, argenx, Johnson & Johnson, and UCB 19 indications currently announced or in development across the anti-FcRn class1
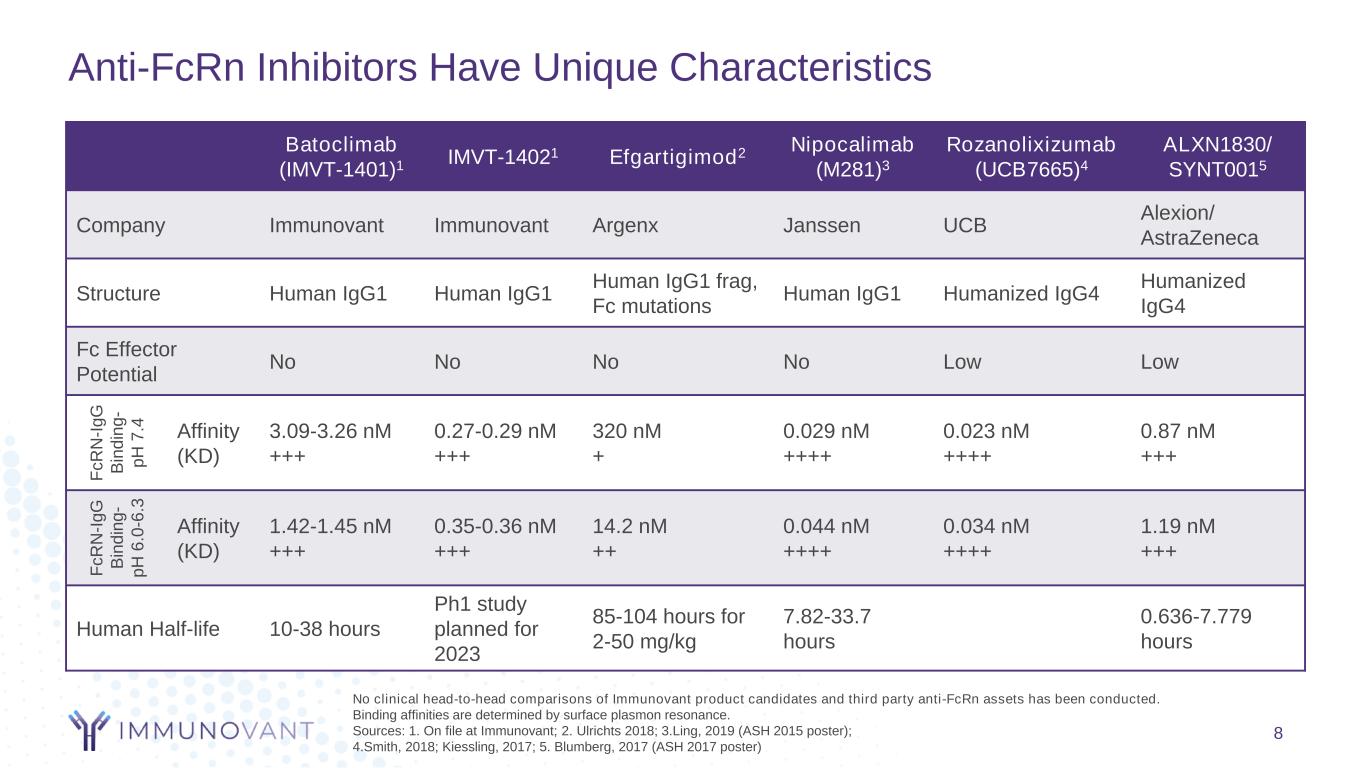
Anti-FcRn Inhibitors Have Unique Characteristics No clinical head-to-head comparisons of Immunovant product candidates and third party anti-FcRn assets has been conducted. Binding affinities are determined by surface plasmon resonance. Sources: 1. On file at Immunovant; 2. Ulrichts 2018; 3.Ling, 2019 (ASH 2015 poster); 4.Smith, 2018; Kiessling, 2017; 5. Blumberg, 2017 (ASH 2017 poster) Batoclimab (IMVT-1401)1 IMVT-14021 Efgartigimod2 Nipocalimab (M281)3 Rozanolixizumab (UCB7665)4 ALXN1830/ SYNT0015 Company Immunovant Immunovant Argenx Janssen UCB Alexion/ AstraZeneca Structure Human IgG1 Human IgG1 Human IgG1 frag, Fc mutations Human IgG1 Humanized IgG4 Humanized IgG4 Fc Effector Potential No No No No Low Low F c R N -I g G B in d in g - p H 7 .4 Affinity (KD) 3.09-3.26 nM +++ 0.27-0.29 nM +++ 320 nM + 0.029 nM ++++ 0.023 nM ++++ 0.87 nM +++ F c R N -I g G B in d in g - p H 6 .0 -6 .3 Affinity (KD) 1.42-1.45 nM +++ 0.35-0.36 nM +++ 14.2 nM ++ 0.044 nM ++++ 0.034 nM ++++ 1.19 nM +++ Human Half-life 10-38 hours Ph1 study planned for 2023 85-104 hours for 2-50 mg/kg 7.82-33.7 hours 0.636-7.779 hours 8
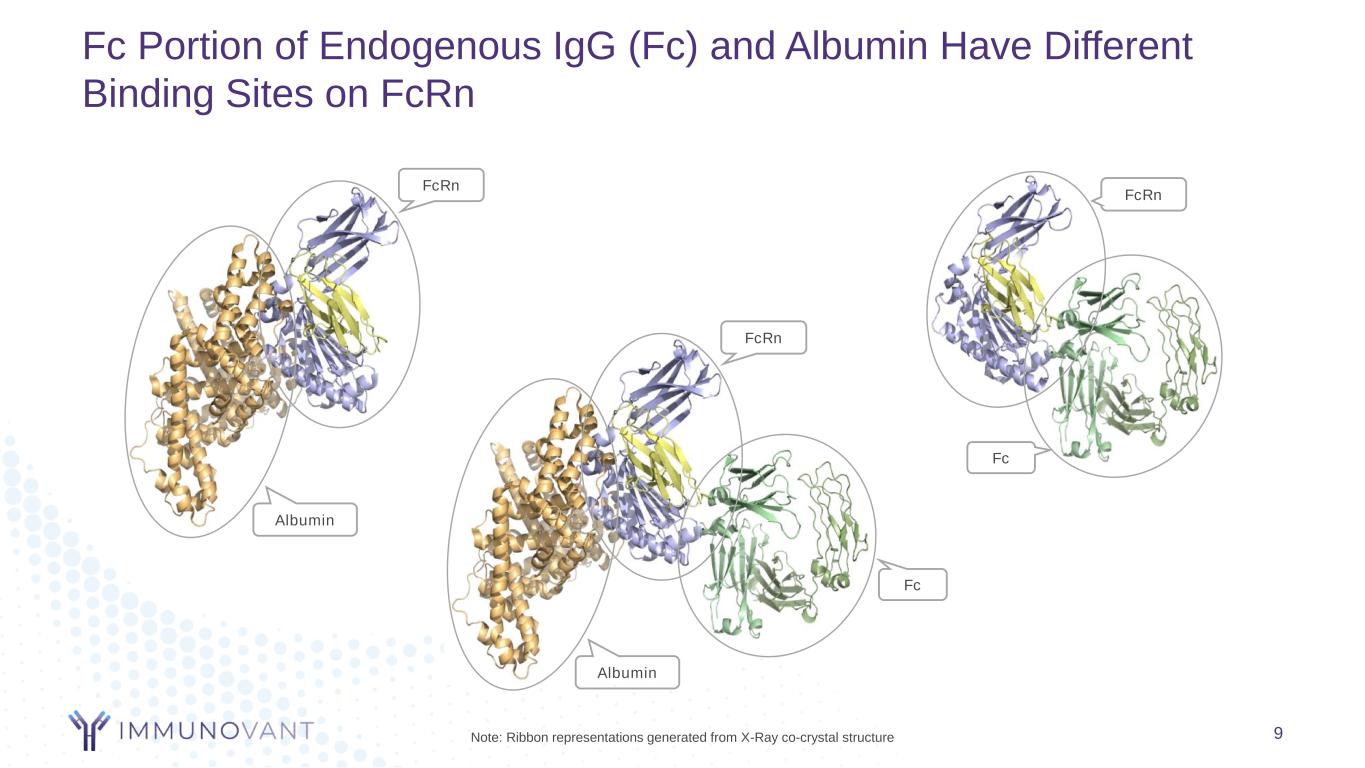
Fc Portion of Endogenous IgG (Fc) and Albumin Have Different Binding Sites on FcRn 9 Albumin FcRn Fc FcRn Albumin FcRn Fc Note: Ribbon representations generated from X-Ray co-crystal structure
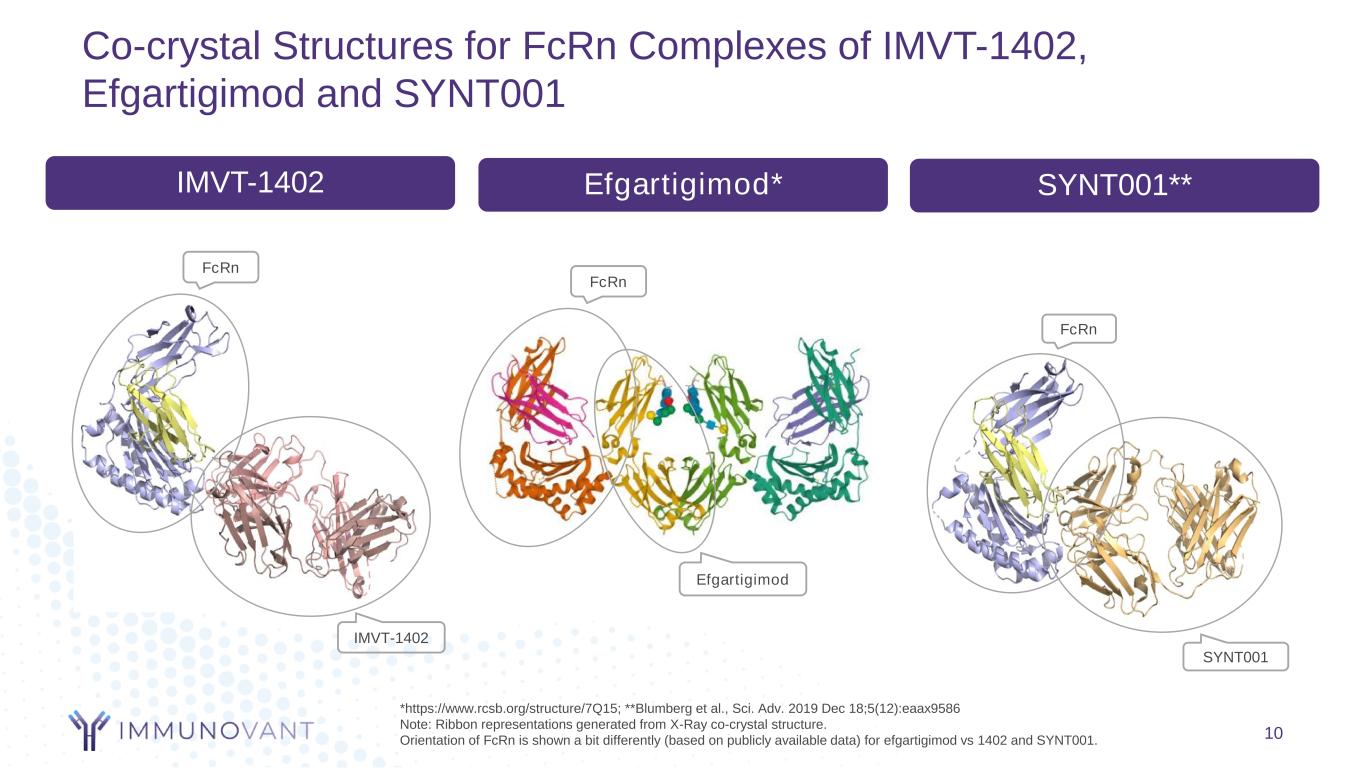
Co-crystal Structures for FcRn Complexes of IMVT-1402, Efgartigimod and SYNT001 10 FcRn SYNT001 IMVT-1402 Efgartigimod* SYNT001** FcRn IMVT-1402 FcRn Efgartigimod *https://www.rcsb.org/structure/7Q15; **Blumberg et al., Sci. Adv. 2019 Dec 18;5(12):eaax9586 Note: Ribbon representations generated from X-Ray co-crystal structure. Orientation of FcRn is shown a bit differently (based on publicly available data) for efgartigimod vs 1402 and SYNT001.
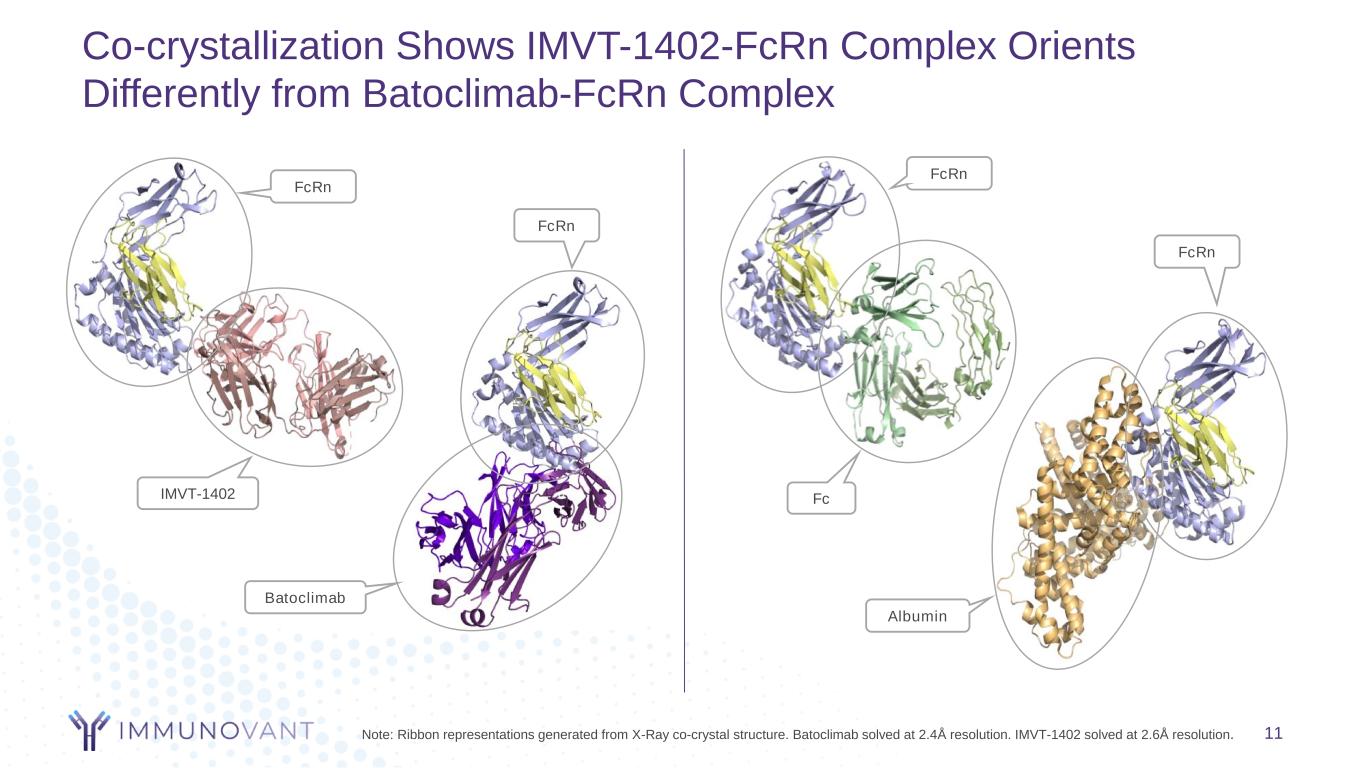
Co-crystallization Shows IMVT-1402-FcRn Complex Orients Differently from Batoclimab-FcRn Complex 11Note: Ribbon representations generated from X-Ray co-crystal structure. Batoclimab solved at 2.4Å resolution. IMVT-1402 solved at 2.6Å resolution. FcRn IMVT-1402 Albumin FcRn Fc FcRn Batoclimab FcRn
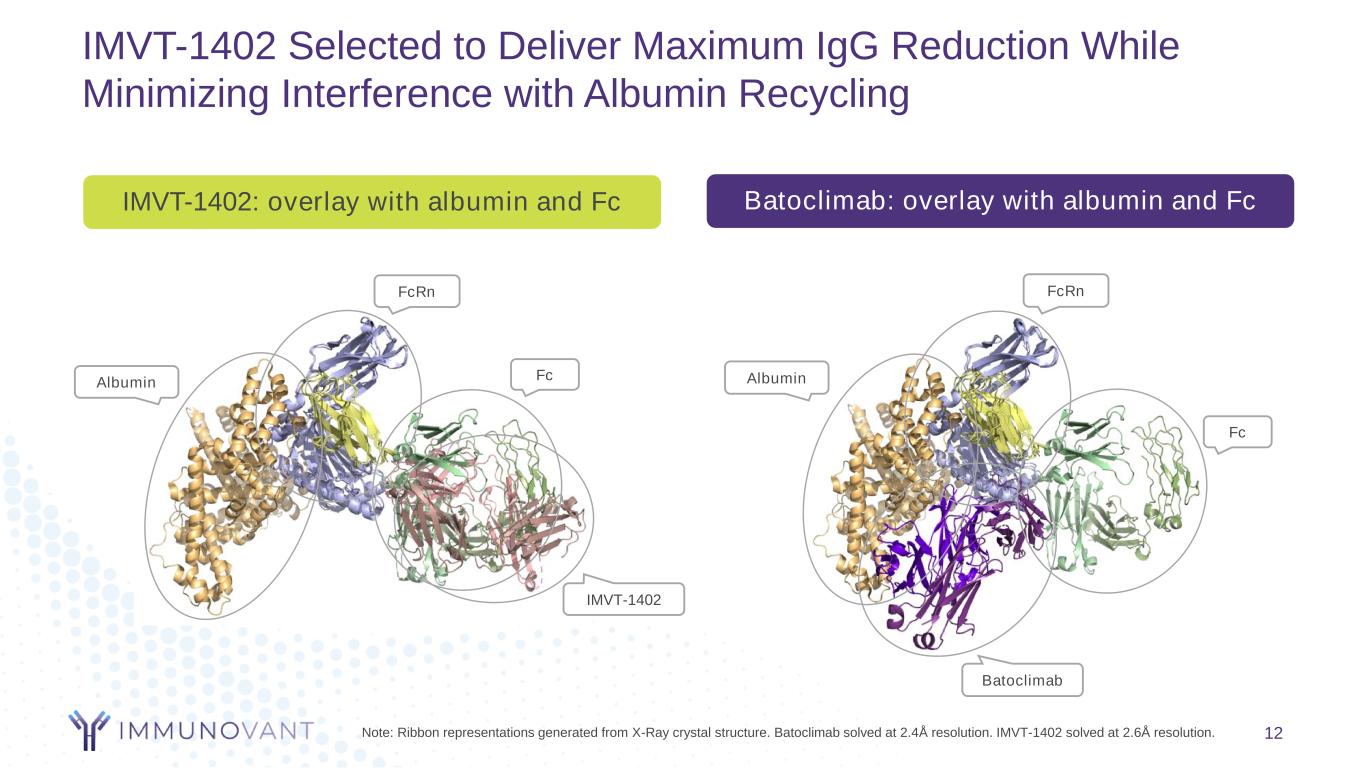
IMVT-1402 Selected to Deliver Maximum IgG Reduction While Minimizing Interference with Albumin Recycling Batoclimab: overlay with albumin and FcIMVT-1402: overlay with albumin and Fc 12Note: Ribbon representations generated from X-Ray crystal structure. Batoclimab solved at 2.4Å resolution. IMVT-1402 solved at 2.6Å resolution. Fc Albumin FcRn Batoclimab Fc Albumin FcRn IMVT-1402
Our Value Proposition: Three Potentially Unique Attributes to Address Unmet Patient Needs 13 % • Strong correlation between deep IgG reduction and increased clinical efficacy Rapid & Deep IgG Reduction • For patients with varying symptom severity and stage of disease Tailored Dosing • To enable self-administration at home Simple subcutaneous injection
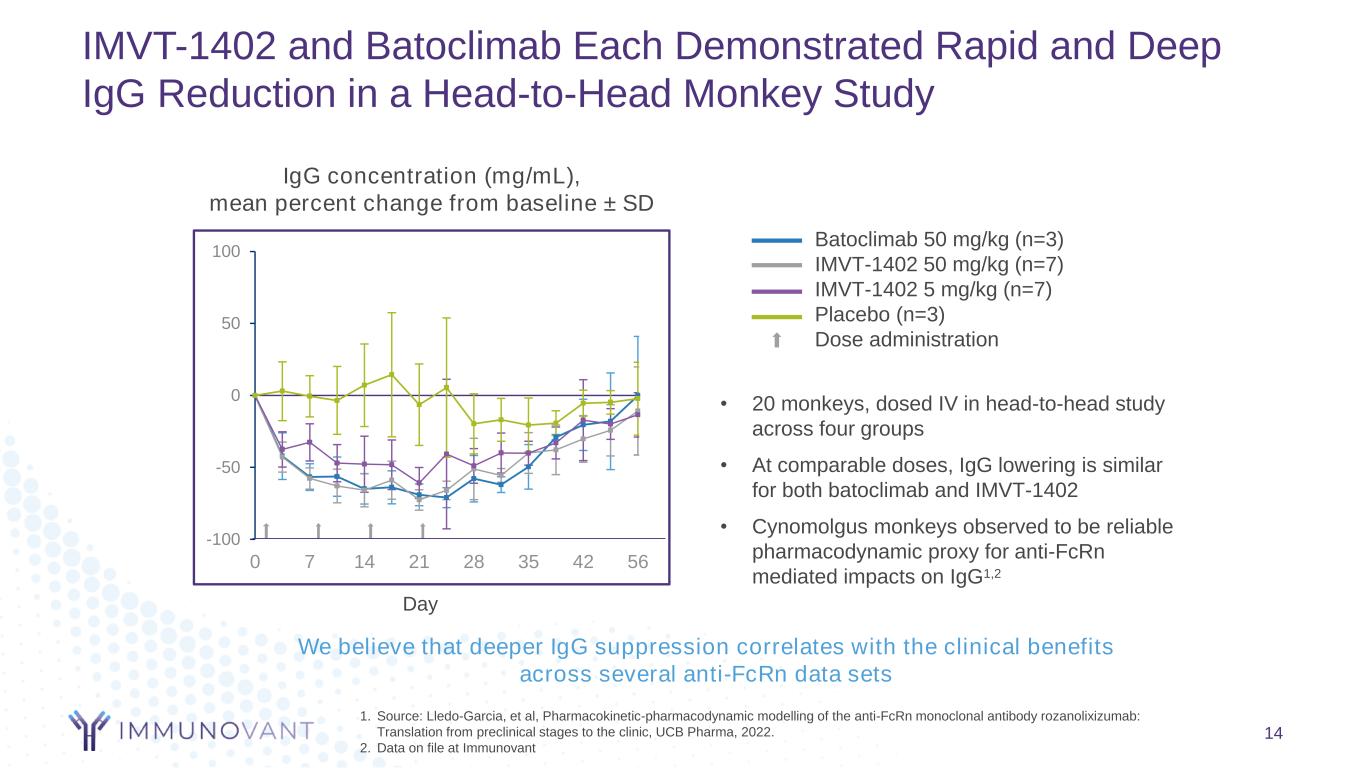
IMVT-1402 and Batoclimab Each Demonstrated Rapid and Deep IgG Reduction in a Head-to-Head Monkey Study 14 1. Source: Lledo-Garcia, et al, Pharmacokinetic-pharmacodynamic modelling of the anti-FcRn monoclonal antibody rozanolixizumab: Translation from preclinical stages to the clinic, UCB Pharma, 2022. 2. Data on file at Immunovant -100 -50 0 50 100 0 7 14 21 28 35 42 56 • 20 monkeys, dosed IV in head-to-head study across four groups • At comparable doses, IgG lowering is similar for both batoclimab and IMVT-1402 • Cynomolgus monkeys observed to be reliable pharmacodynamic proxy for anti-FcRn mediated impacts on IgG1,2 IgG concentration (mg/mL), mean percent change from baseline ± SD Day Batoclimab 50 mg/kg (n=3) IMVT-1402 50 mg/kg (n=7) IMVT-1402 5 mg/kg (n=7) Placebo (n=3) Dose administration We believe that deeper IgG suppression correlates with the clinical benefits across several anti-FcRn data sets
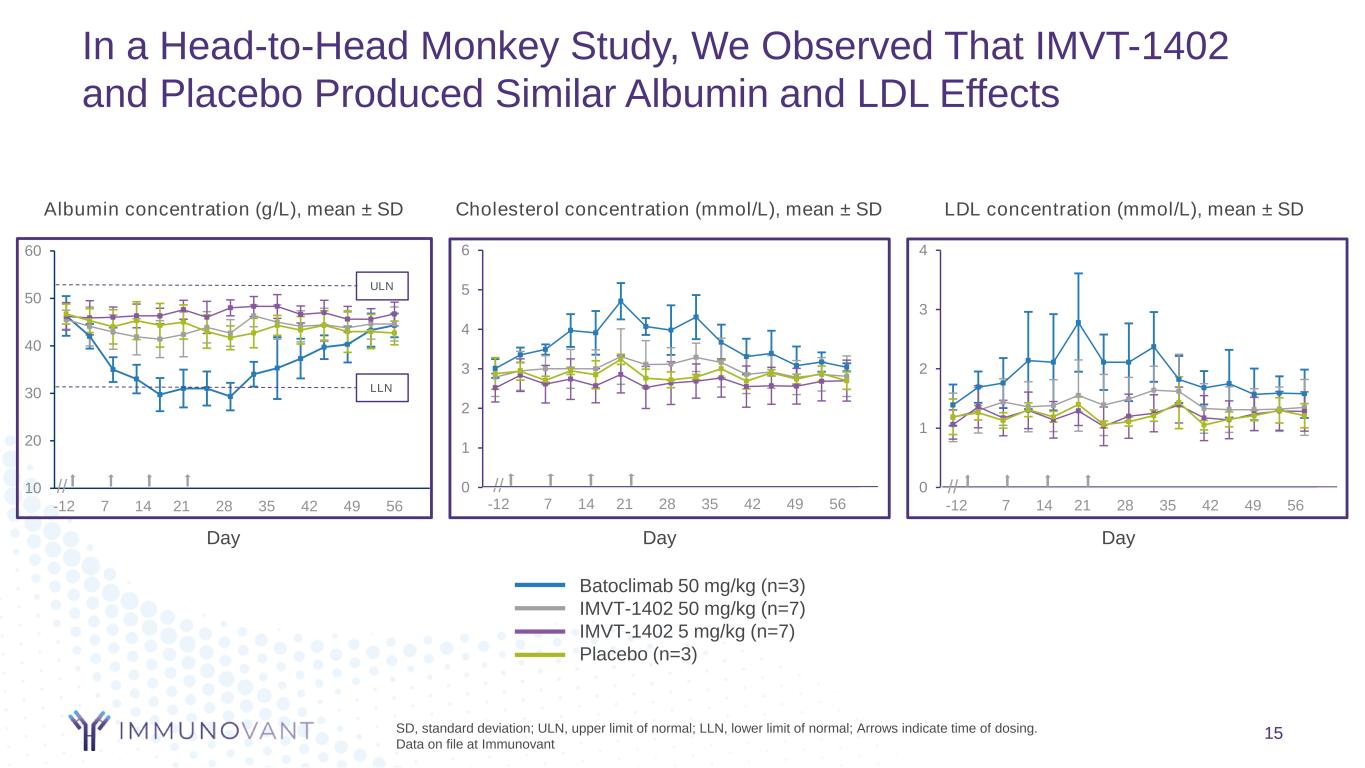
15 In a Head-to-Head Monkey Study, We Observed That IMVT-1402 and Placebo Produced Similar Albumin and LDL Effects Day SD, standard deviation; ULN, upper limit of normal; LLN, lower limit of normal; Arrows indicate time of dosing. Data on file at Immunovant Day Batoclimab 50 mg/kg (n=3) IMVT-1402 50 mg/kg (n=7) IMVT-1402 5 mg/kg (n=7) Placebo (n=3) LDL concentration (mmol/L), mean ± SDAlbumin concentration (g/L), mean ± SD -12 7 14 21 28 35 42 49 56 0 1 2 3 4 //10 20 30 40 50 60 ULN LLN -12 7 14 21 28 35 42 49 56 // Day -12 7 14 21 28 35 42 49 56 // Cholesterol concentration (mmol/L), mean ± SD 0 1 2 3 4 5 6
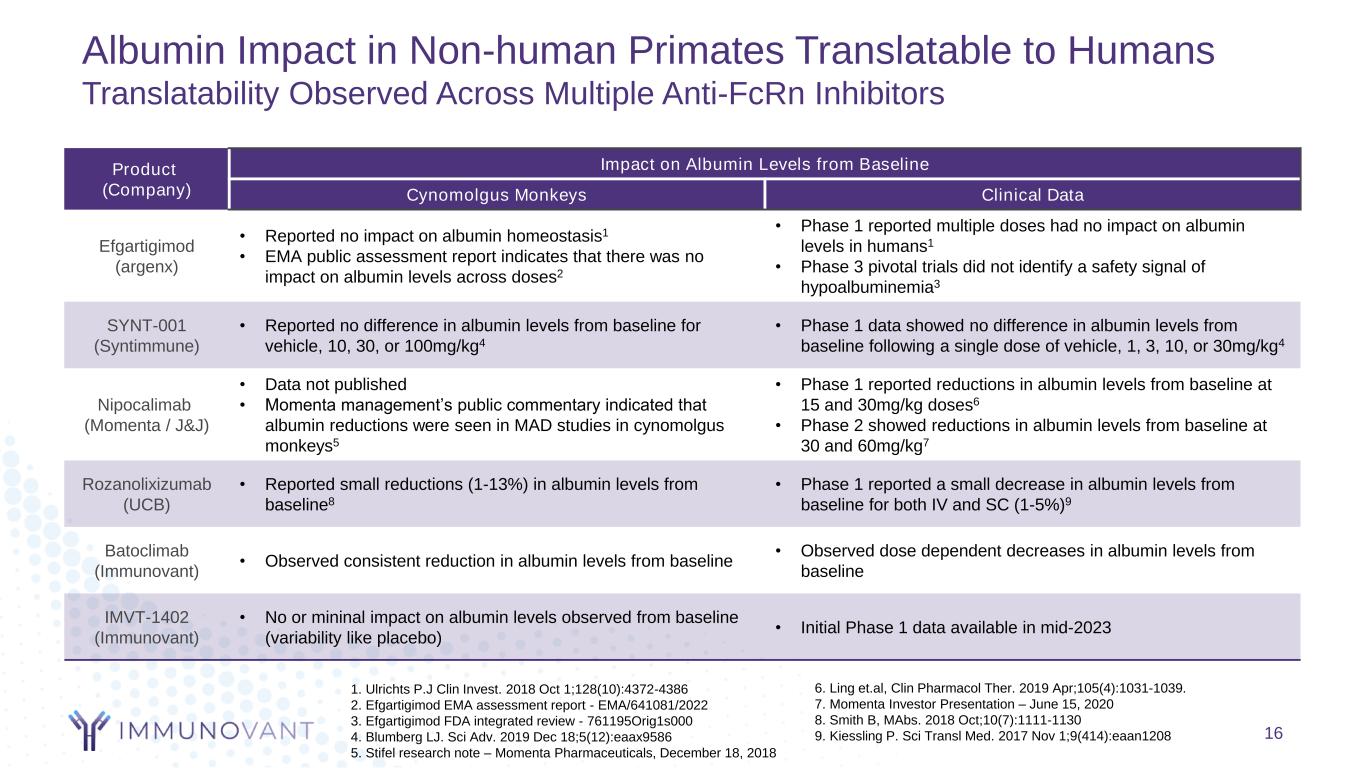
Albumin Impact in Non-human Primates Translatable to Humans Translatability Observed Across Multiple Anti-FcRn Inhibitors 16 1. Ulrichts P.J Clin Invest. 2018 Oct 1;128(10):4372-4386 2. Efgartigimod EMA assessment report - EMA/641081/2022 3. Efgartigimod FDA integrated review - 761195Orig1s000 4. Blumberg LJ. Sci Adv. 2019 Dec 18;5(12):eaax9586 5. Stifel research note – Momenta Pharmaceuticals, December 18, 2018 6. Ling et.al, Clin Pharmacol Ther. 2019 Apr;105(4):1031-1039. 7. Momenta Investor Presentation – June 15, 2020 8. Smith B, MAbs. 2018 Oct;10(7):1111-1130 9. Kiessling P. Sci Transl Med. 2017 Nov 1;9(414):eaan1208 Product (Company) Impact on Albumin Levels from Baseline Cynomolgus Monkeys Clinical Data Efgartigimod (argenx) • Reported no impact on albumin homeostasis1 • EMA public assessment report indicates that there was no impact on albumin levels across doses2 • Phase 1 reported multiple doses had no impact on albumin levels in humans1 • Phase 3 pivotal trials did not identify a safety signal of hypoalbuminemia3 SYNT-001 (Syntimmune) • Reported no difference in albumin levels from baseline for vehicle, 10, 30, or 100mg/kg4 • Phase 1 data showed no difference in albumin levels from baseline following a single dose of vehicle, 1, 3, 10, or 30mg/kg4 Nipocalimab (Momenta / J&J) • Data not published • Momenta management’s public commentary indicated that albumin reductions were seen in MAD studies in cynomolgus monkeys5 • Phase 1 reported reductions in albumin levels from baseline at 15 and 30mg/kg doses6 • Phase 2 showed reductions in albumin levels from baseline at 30 and 60mg/kg7 Rozanolixizumab (UCB) • Reported small reductions (1-13%) in albumin levels from baseline8 • Phase 1 reported a small decrease in albumin levels from baseline for both IV and SC (1-5%)9 Batoclimab (Immunovant) • Observed consistent reduction in albumin levels from baseline • Observed dose dependent decreases in albumin levels from baseline IMVT-1402 (Immunovant) • No or mininal impact on albumin levels observed from baseline (variability like placebo) • Initial Phase 1 data available in mid-2023
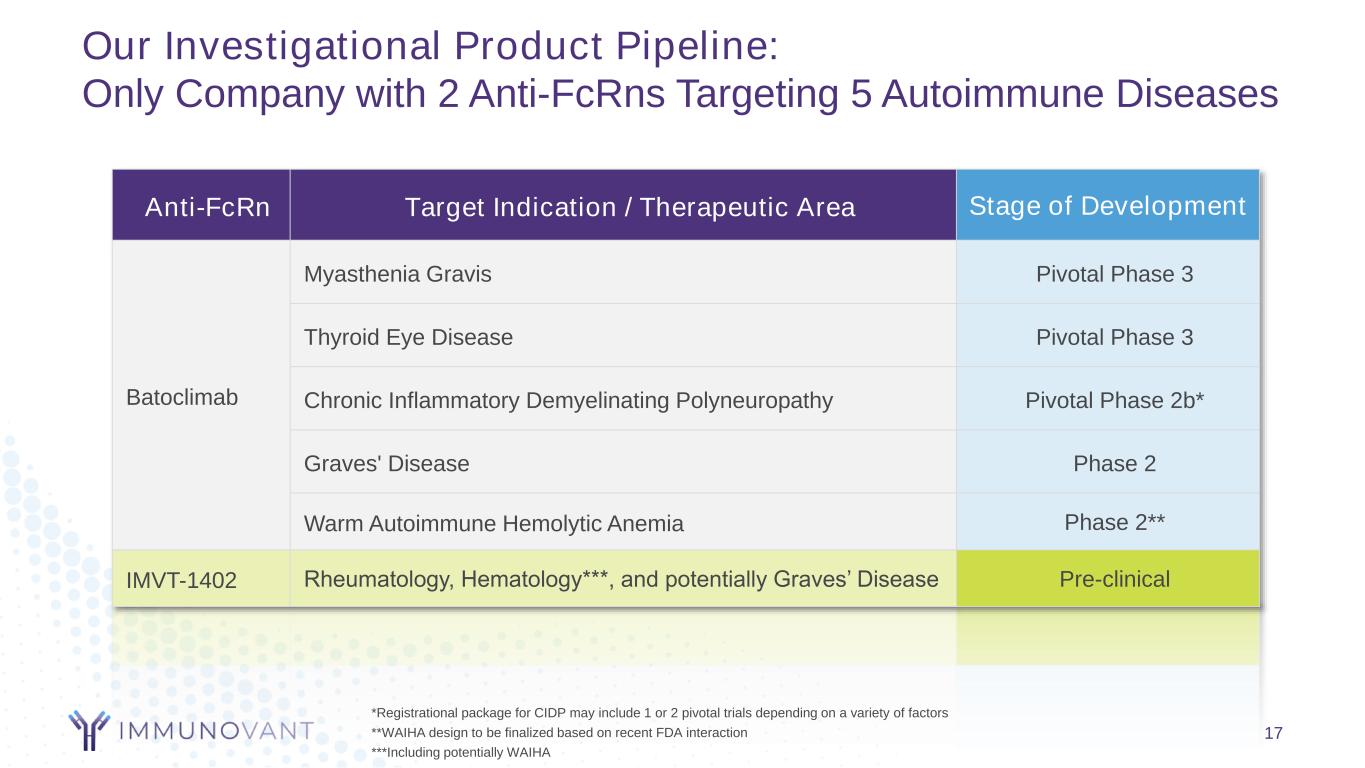
Our Investigational Product Pipeline: Only Company with 2 Anti-FcRns Targeting 5 Autoimmune Diseases 17 Anti-FcRn Target Indication / Therapeutic Area Stage of Development Batoclimab Myasthenia Gravis Pivotal Phase 3 Thyroid Eye Disease Pivotal Phase 3 Chronic Inflammatory Demyelinating Polyneuropathy Pivotal Phase 2b* Graves' Disease Phase 2 Warm Autoimmune Hemolytic Anemia Phase 2** IMVT-1402 Rheumatology, Hematology***, and potentially Graves’ Disease Pre-clinical *Registrational package for CIDP may include 1 or 2 pivotal trials depending on a variety of factors **WAIHA design to be finalized based on recent FDA interaction ***Including potentially WAIHA

Myasthenia Gravis
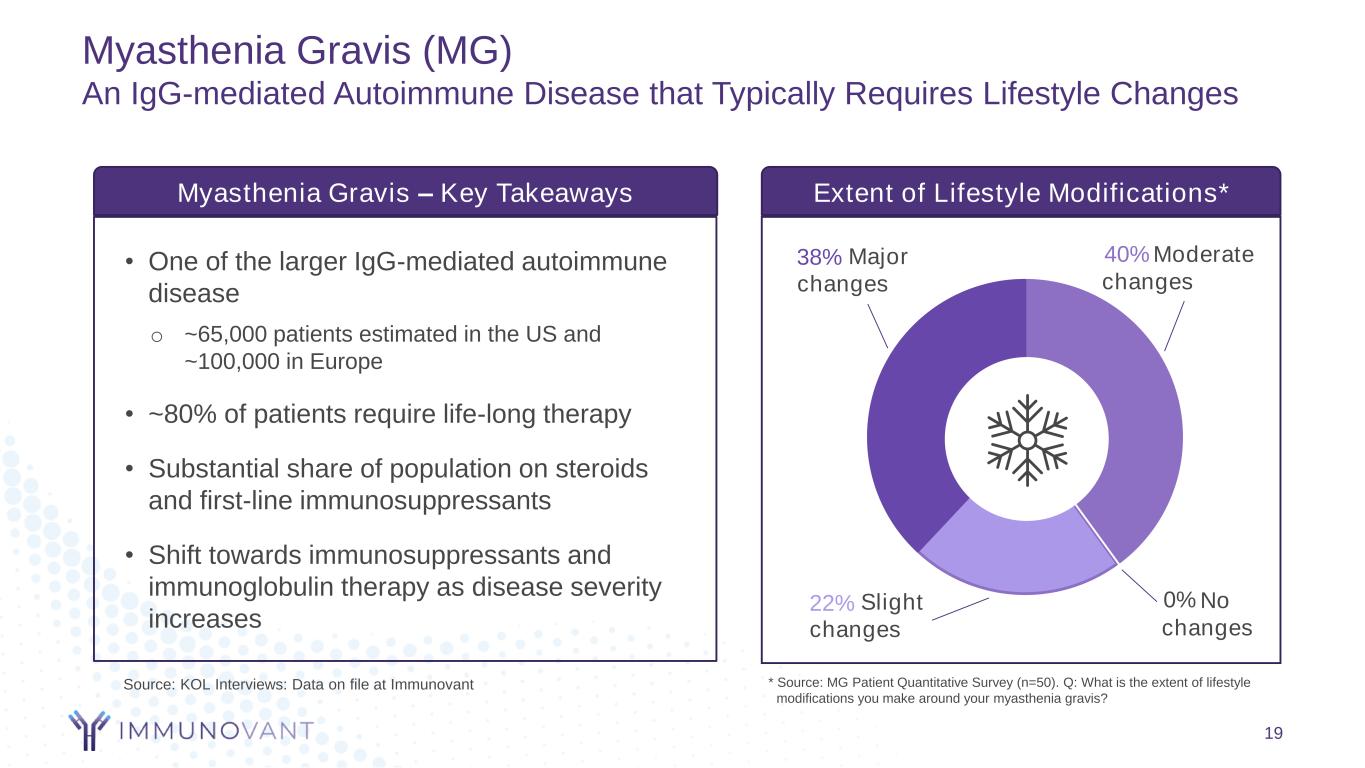
Myasthenia Gravis (MG) An IgG-mediated Autoimmune Disease that Typically Requires Lifestyle Changes 19 • One of the larger IgG-mediated autoimmune disease o ~65,000 patients estimated in the US and ~100,000 in Europe • ~80% of patients require life-long therapy • Substantial share of population on steroids and first-line immunosuppressants • Shift towards immunosuppressants and immunoglobulin therapy as disease severity increases Myasthenia Gravis – Key Takeaways Extent of Lifestyle Modifications* * Source: MG Patient Quantitative Survey (n=50). Q: What is the extent of lifestyle modifications you make around your myasthenia gravis? Major changes 38% Moderate changes 40% Slight changes 22% No changes 0% Source: KOL Interviews: Data on file at Immunovant
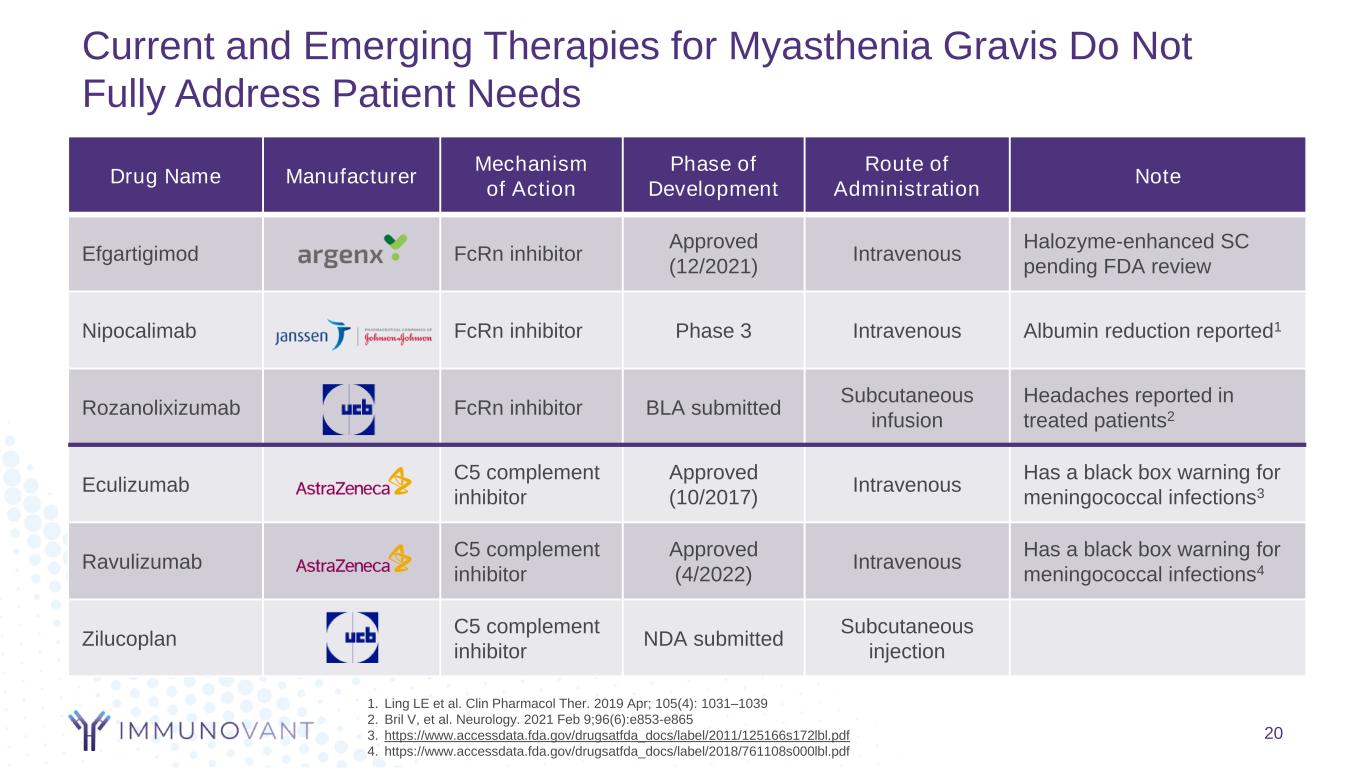
Drug Name Manufacturer Mechanism of Action Phase of Development Route of Administration Note Efgartigimod FcRn inhibitor Approved (12/2021) Intravenous Halozyme-enhanced SC pending FDA review Nipocalimab FcRn inhibitor Phase 3 Intravenous Albumin reduction reported1 Rozanolixizumab FcRn inhibitor BLA submitted Subcutaneous infusion Headaches reported in treated patients2 Eculizumab C5 complement inhibitor Approved (10/2017) Intravenous Has a black box warning for meningococcal infections3 Ravulizumab C5 complement inhibitor Approved (4/2022) Intravenous Has a black box warning for meningococcal infections4 Zilucoplan C5 complement inhibitor NDA submitted Subcutaneous injection Current and Emerging Therapies for Myasthenia Gravis Do Not Fully Address Patient Needs 1. Ling LE et al. Clin Pharmacol Ther. 2019 Apr; 105(4): 1031–1039 2. Bril V, et al. Neurology. 2021 Feb 9;96(6):e853-e865 3. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125166s172lbl.pdf 4. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761108s000lbl.pdf 20
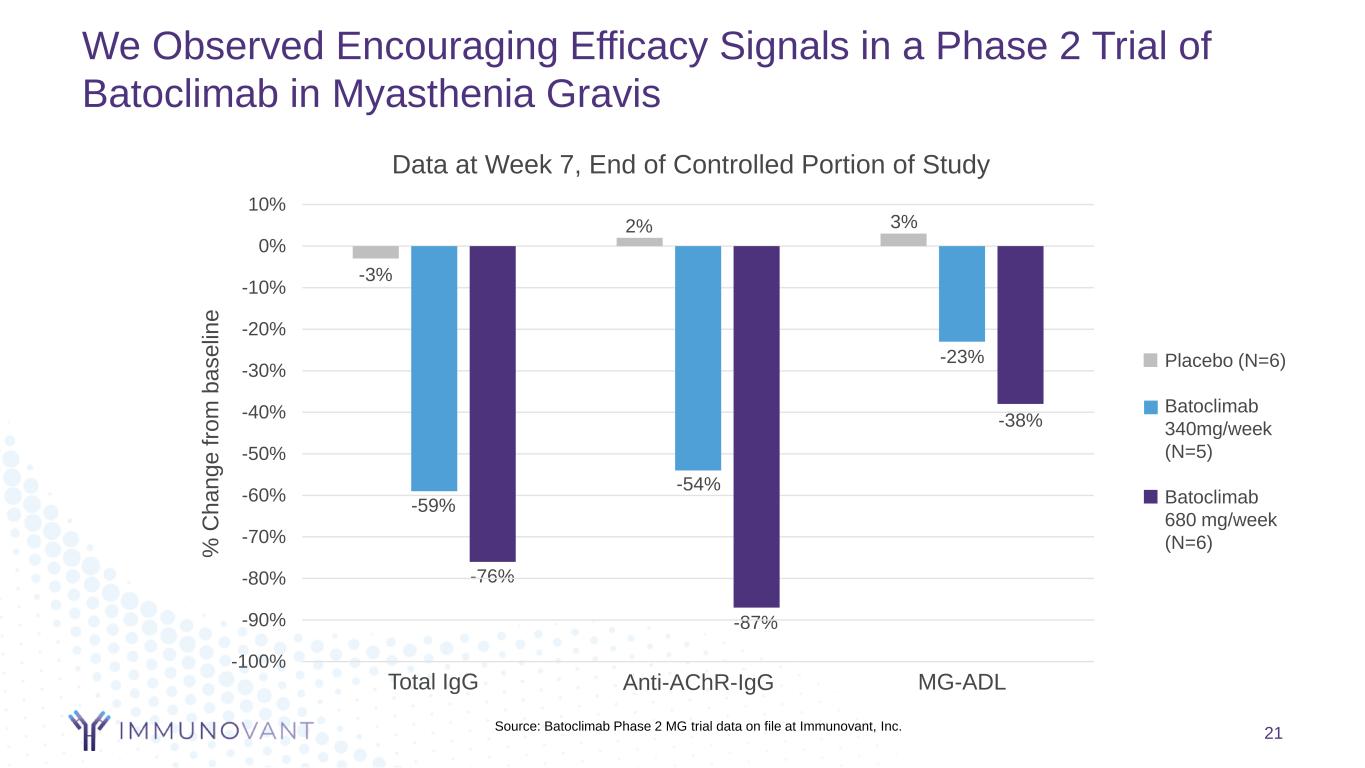
We Observed Encouraging Efficacy Signals in a Phase 2 Trial of Batoclimab in Myasthenia Gravis 21 -3% -59% 2% 3% -23% -3% -38% -76% -54% -87% -100% -90% -80% -70% -60% -50% -40% -30% -20% -10% 0% 10% Data at Week 7, End of Controlled Portion of Study Placebo (N=6) Batoclimab 340mg/week (N=5) Batoclimab 680 mg/week (N=6) Anti-AChR-IgGTotal IgG MG-ADL % C h a n g e f ro m b a s e lin e Source: Batoclimab Phase 2 MG trial data on file at Immunovant, Inc.
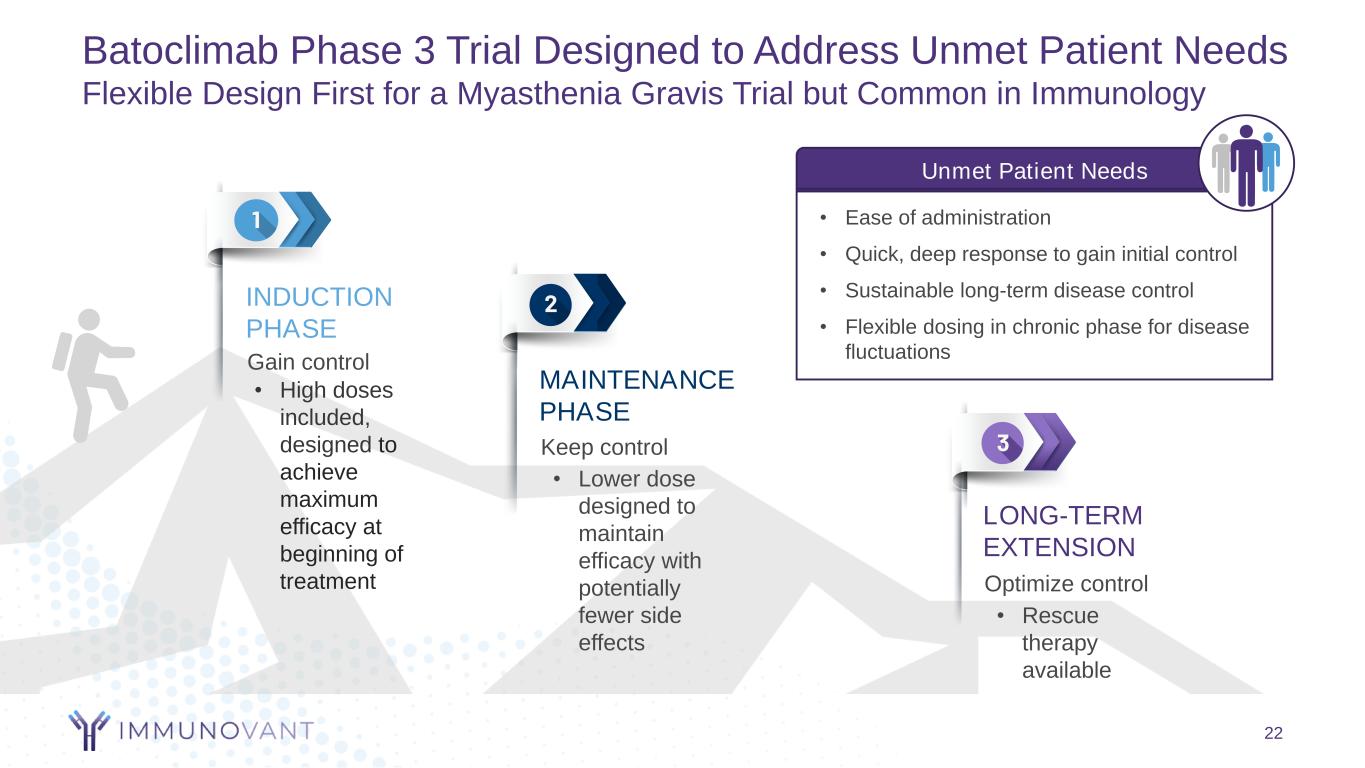
Batoclimab Phase 3 Trial Designed to Address Unmet Patient Needs Flexible Design First for a Myasthenia Gravis Trial but Common in Immunology 22 Keep control MAINTENANCE PHASE Optimize control LONG-TERM EXTENSION Gain control INDUCTION PHASE • High doses included, designed to achieve maximum efficacy at beginning of treatment • Lower dose designed to maintain efficacy with potentially fewer side effects • Rescue therapy available Unmet Patient Needs • Ease of administration • Quick, deep response to gain initial control • Sustainable long-term disease control • Flexible dosing in chronic phase for disease fluctuations
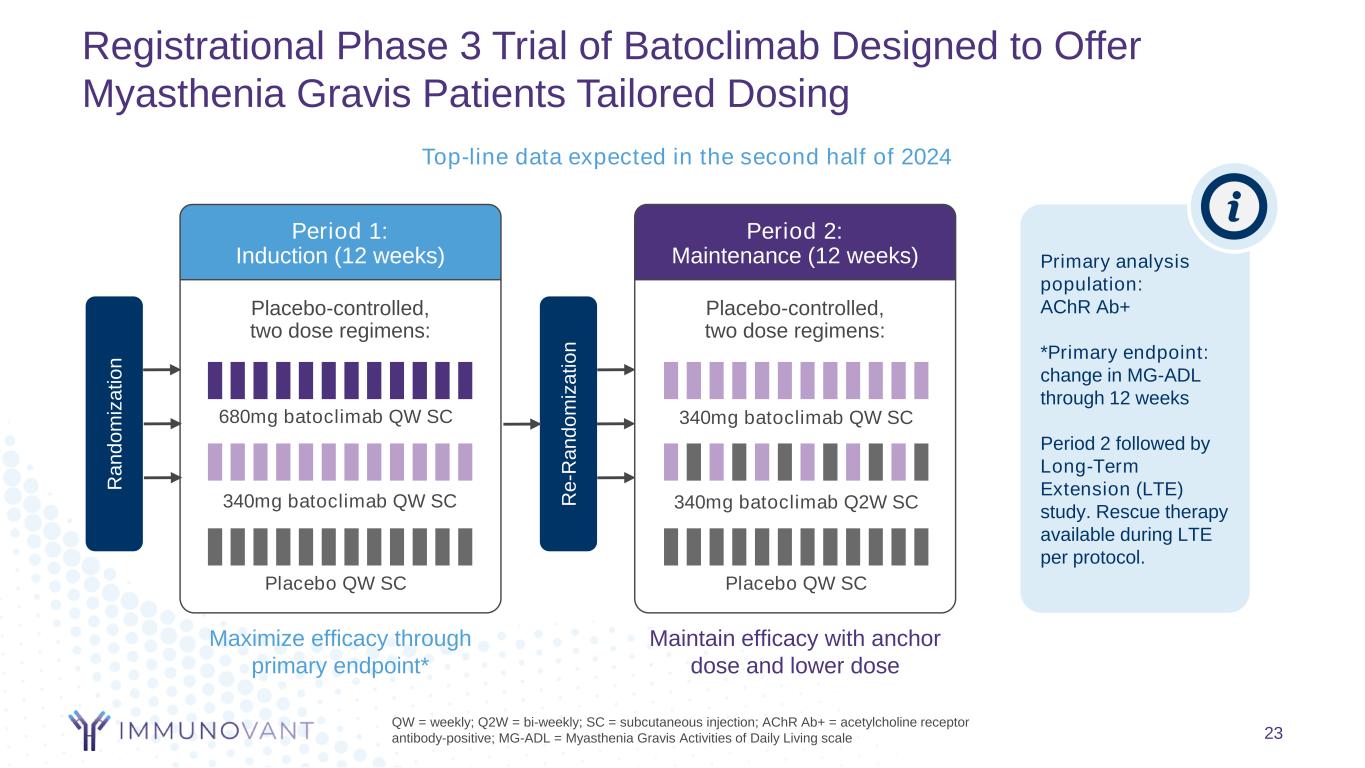
Registrational Phase 3 Trial of Batoclimab Designed to Offer Myasthenia Gravis Patients Tailored Dosing 23 QW = weekly; Q2W = bi-weekly; SC = subcutaneous injection; AChR Ab+ = acetylcholine receptor antibody-positive; MG-ADL = Myasthenia Gravis Activities of Daily Living scale Placebo-controlled, two dose regimens: Period 1: Induction (12 weeks) Maximize efficacy through primary endpoint* Placebo-controlled, two dose regimens: Period 2: Maintenance (12 weeks) Maintain efficacy with anchor dose and lower dose Placebo QW SC Placebo QW SC 340mg batoclimab QW SC 680mg batoclimab QW SC 340mg batoclimab Q2W SC 340mg batoclimab QW SC Primary analysis population: AChR Ab+ *Primary endpoint: change in MG-ADL through 12 weeks Period 2 followed by Long-Term Extension (LTE) study. Rescue therapy available during LTE per protocol. R a n d o m iz a ti o n R e -R a n d o m iz a ti o n Top-line data expected in the second half of 2024
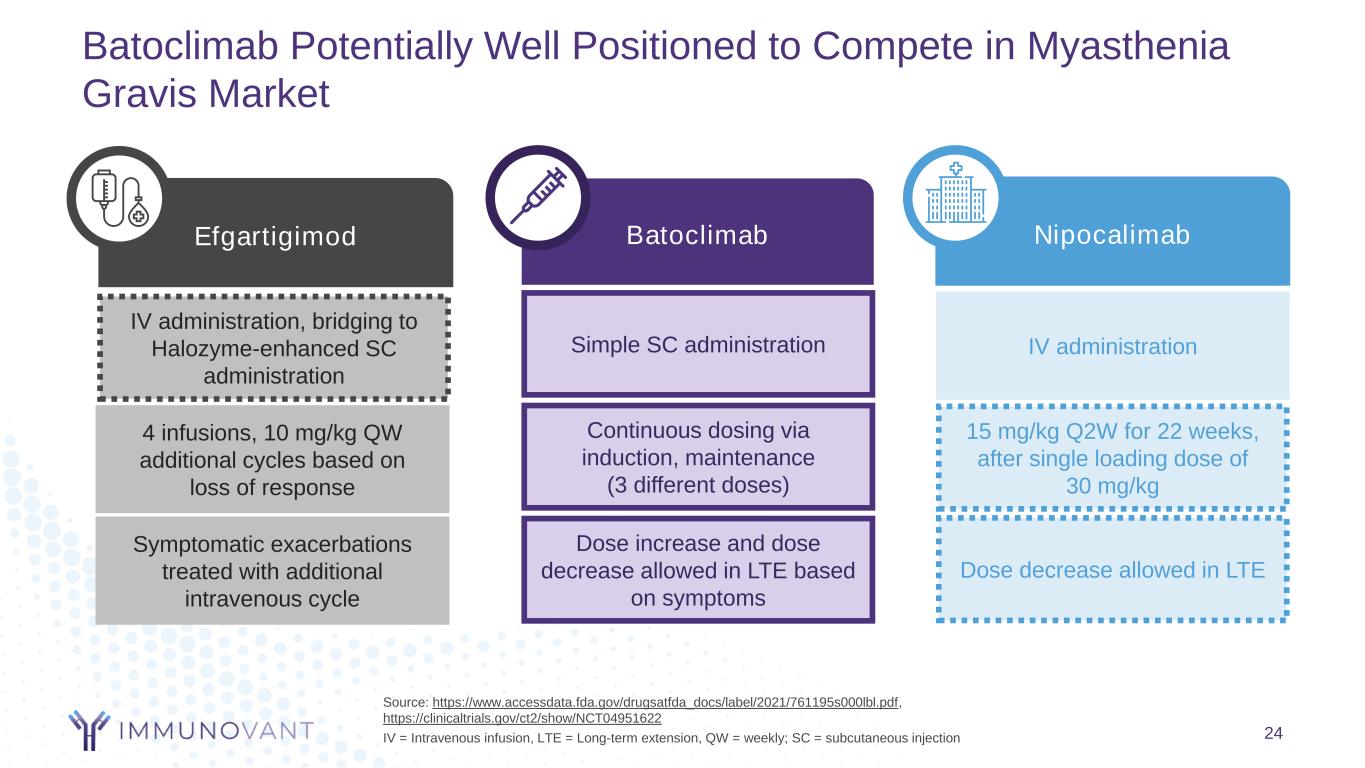
24 Batoclimab Potentially Well Positioned to Compete in Myasthenia Gravis Market Continuous dosing via induction, maintenance (3 different doses) Dose increase and dose decrease allowed in LTE based on symptoms Simple SC administration Batoclimab IV administration, bridging to Halozyme-enhanced SC administration 4 infusions, 10 mg/kg QW additional cycles based on loss of response Symptomatic exacerbations treated with additional intravenous cycle Efgartigimod 15 mg/kg Q2W for 22 weeks, after single loading dose of 30 mg/kg IV administration Dose decrease allowed in LTE Nipocalimab Source: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761195s000lbl.pdf, https://clinicaltrials.gov/ct2/show/NCT04951622 IV = Intravenous infusion, LTE = Long-term extension, QW = weekly; SC = subcutaneous injection

Thyroid Eye Disease
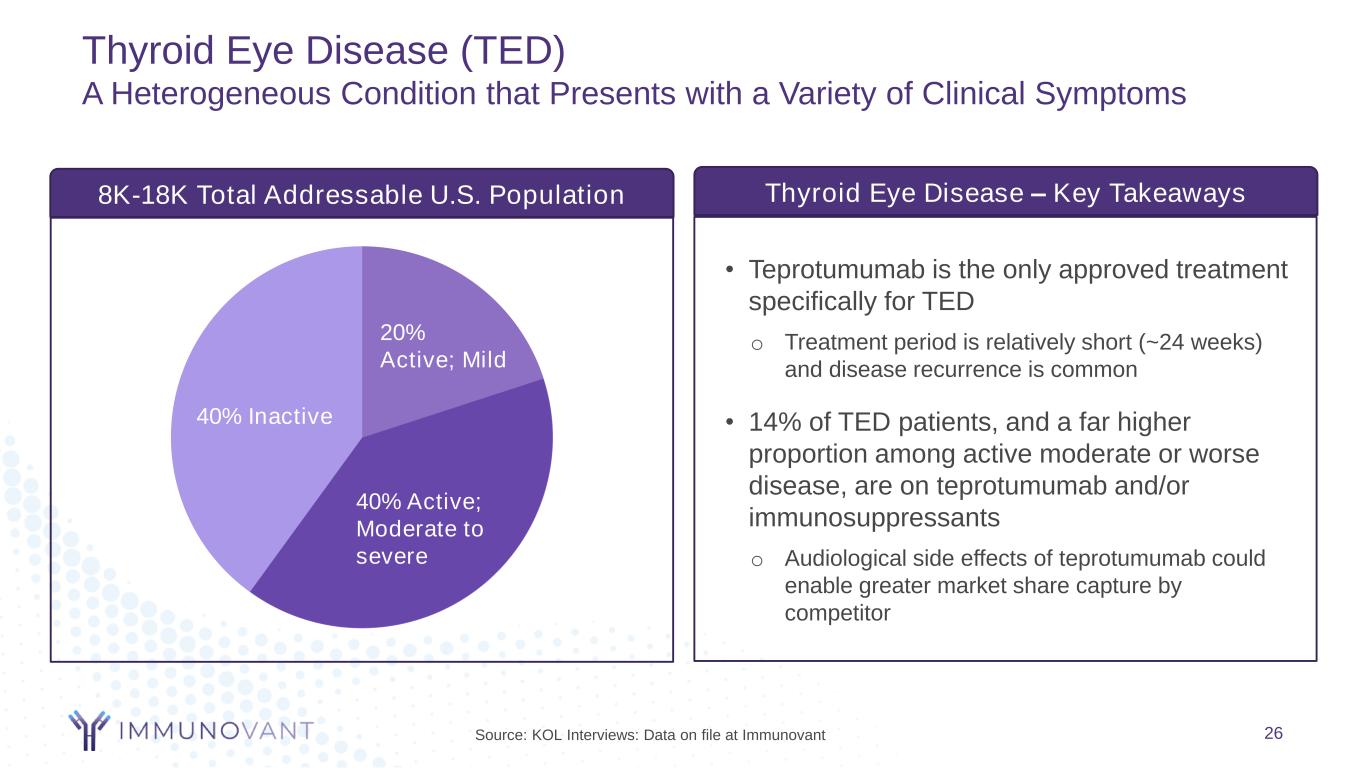
Thyroid Eye Disease (TED) A Heterogeneous Condition that Presents with a Variety of Clinical Symptoms 26 • Teprotumumab is the only approved treatment specifically for TED o Treatment period is relatively short (~24 weeks) and disease recurrence is common • 14% of TED patients, and a far higher proportion among active moderate or worse disease, are on teprotumumab and/or immunosuppressants o Audiological side effects of teprotumumab could enable greater market share capture by competitor Thyroid Eye Disease – Key Takeaways 8K-18K Total Addressable U.S. Population 40% Inactive 20% Active; Mild 40% Active; Moderate to severe Source: KOL Interviews: Data on file at Immunovant
27 Unique Dynamics of TED Market Create Potentially Favorable Commercial Opportunity for New Therapeutic Approaches Reimbursement is often strictly to label for specialty products TED products will likely continue to be labeled for a fixed duration equal to the controlled period of the registration trials In the OPTIC 48-week off-treatment follow-up period1, 44% of teprotumumab patients who were proptosis responders at Week 24 in OPTIC were not proptosis responders at Week 72 illustrating the opportunity for additional treatment We anticipate that patients who do not maintain their proptosis response will be candidates for a new mechanism of action We believe that a simple subcutaneous route of administration is also important to patients, and perhaps more so during retreatment due to total duration 1. Horizon Therapeutics, 2020, New Topline TEPEZZA® (teprotumumab-trbw) Data Underscore its Efficacy in Longer Disease Duration, Long-Term Durability and Potential for Retreatment in People Living with Thyroid Eye Disease (TED)
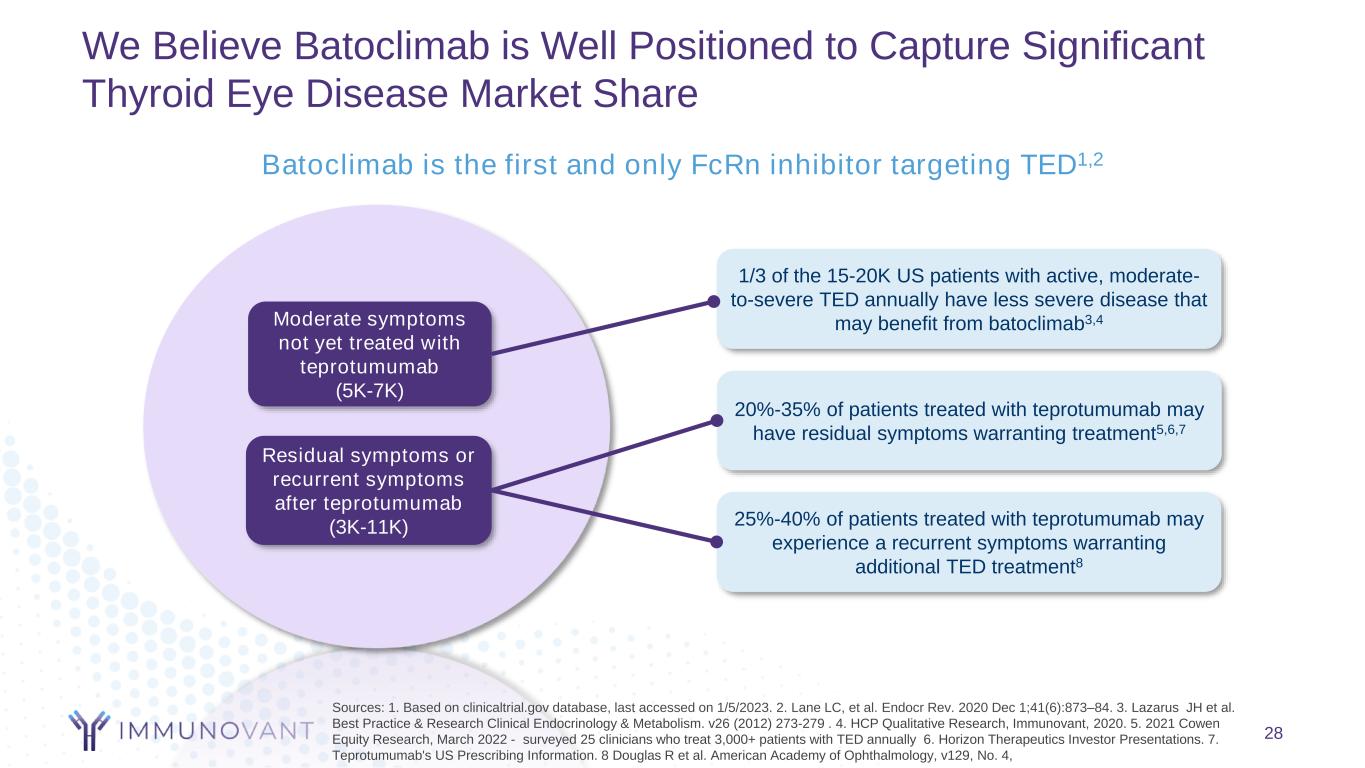
28 Batoclimab is the first and only FcRn inhibitor targeting TED1,2 Residual symptoms or recurrent symptoms after teprotumumab (3K-11K) Moderate symptoms not yet treated with teprotumumab (5K-7K) 1/3 of the 15-20K US patients with active, moderate- to-severe TED annually have less severe disease that may benefit from batoclimab3,4 20%-35% of patients treated with teprotumumab may have residual symptoms warranting treatment5,6,7 25%-40% of patients treated with teprotumumab may experience a recurrent symptoms warranting additional TED treatment8 We Believe Batoclimab is Well Positioned to Capture Significant Thyroid Eye Disease Market Share Sources: 1. Based on clinicaltrial.gov database, last accessed on 1/5/2023. 2. Lane LC, et al. Endocr Rev. 2020 Dec 1;41(6):873–84. 3. Lazarus JH et al. Best Practice & Research Clinical Endocrinology & Metabolism. v26 (2012) 273-279 . 4. HCP Qualitative Research, Immunovant, 2020. 5. 2021 Cowen Equity Research, March 2022 - surveyed 25 clinicians who treat 3,000+ patients with TED annually 6. Horizon Therapeutics Investor Presentations. 7. Teprotumumab's US Prescribing Information. 8 Douglas R et al. American Academy of Ophthalmology, v129, No. 4,
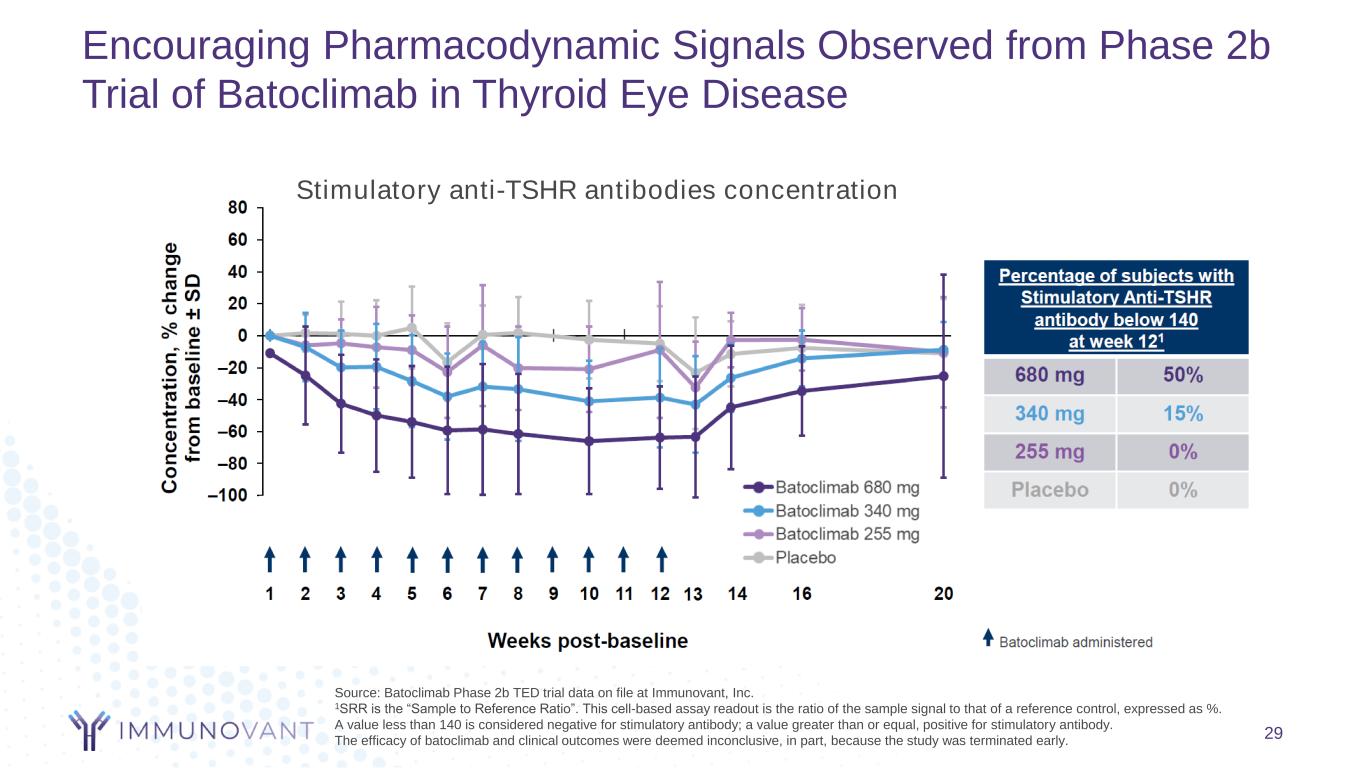
Encouraging Pharmacodynamic Signals Observed from Phase 2b Trial of Batoclimab in Thyroid Eye Disease Source: Batoclimab Phase 2b TED trial data on file at Immunovant, Inc. 1SRR is the “Sample to Reference Ratio”. This cell-based assay readout is the ratio of the sample signal to that of a reference control, expressed as %. A value less than 140 is considered negative for stimulatory antibody; a value greater than or equal, positive for stimulatory antibody. The efficacy of batoclimab and clinical outcomes were deemed inconclusive, in part, because the study was terminated early. Stimulatory anti-TSHR antibodies concentration 29
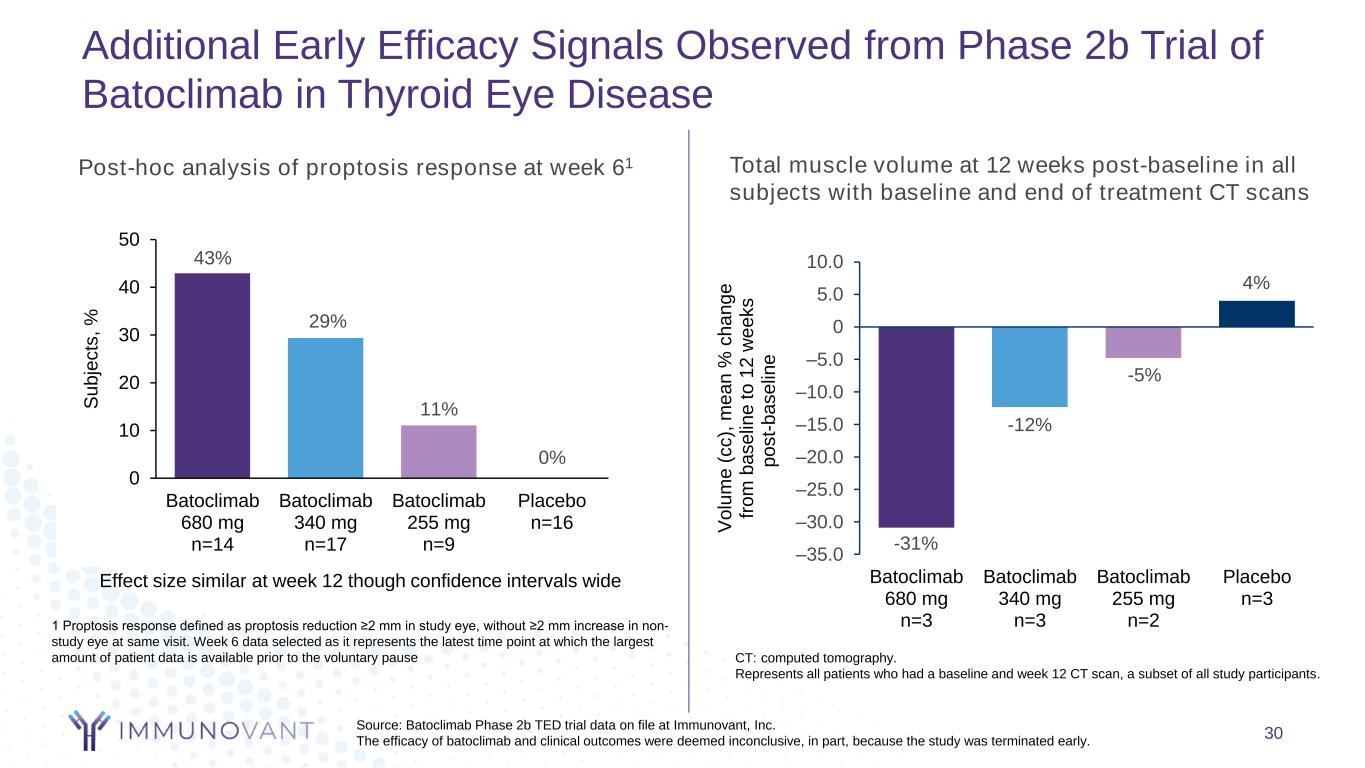
1 Proptosis response defined as proptosis reduction ≥2 mm in study eye, without ≥2 mm increase in non- study eye at same visit. Week 6 data selected as it represents the latest time point at which the largest amount of patient data is available prior to the voluntary pause –35.0 –30.0 –25.0 –20.0 –15.0 –10.0 –5.0 0 5.0 10.0 Batoclimab 680 mg n=3 Batoclimab 340 mg n=3 Batoclimab 255 mg n=2 Placebo n=3 V o lu m e ( c c ), m e a n % c h a n g e fr o m b a s e lin e t o 1 2 w e e k s p o s t- b a s e lin e Total muscle volume at 12 weeks post-baseline in all subjects with baseline and end of treatment CT scans CT: computed tomography. Represents all patients who had a baseline and week 12 CT scan, a subset of all study participants. Additional Early Efficacy Signals Observed from Phase 2b Trial of Batoclimab in Thyroid Eye Disease Source: Batoclimab Phase 2b TED trial data on file at Immunovant, Inc. The efficacy of batoclimab and clinical outcomes were deemed inconclusive, in part, because the study was terminated early. 0 0 10 20 30 40 50 Batoclimab 680 mg n=14 Batoclimab 340 mg n=17 Batoclimab 255 mg n=9 Placebo n=16 S u b je c ts , % Effect size similar at week 12 though confidence intervals wide Post-hoc analysis of proptosis response at week 61 11% 29% 43% 0% 30 -12% 4% -31% -5%
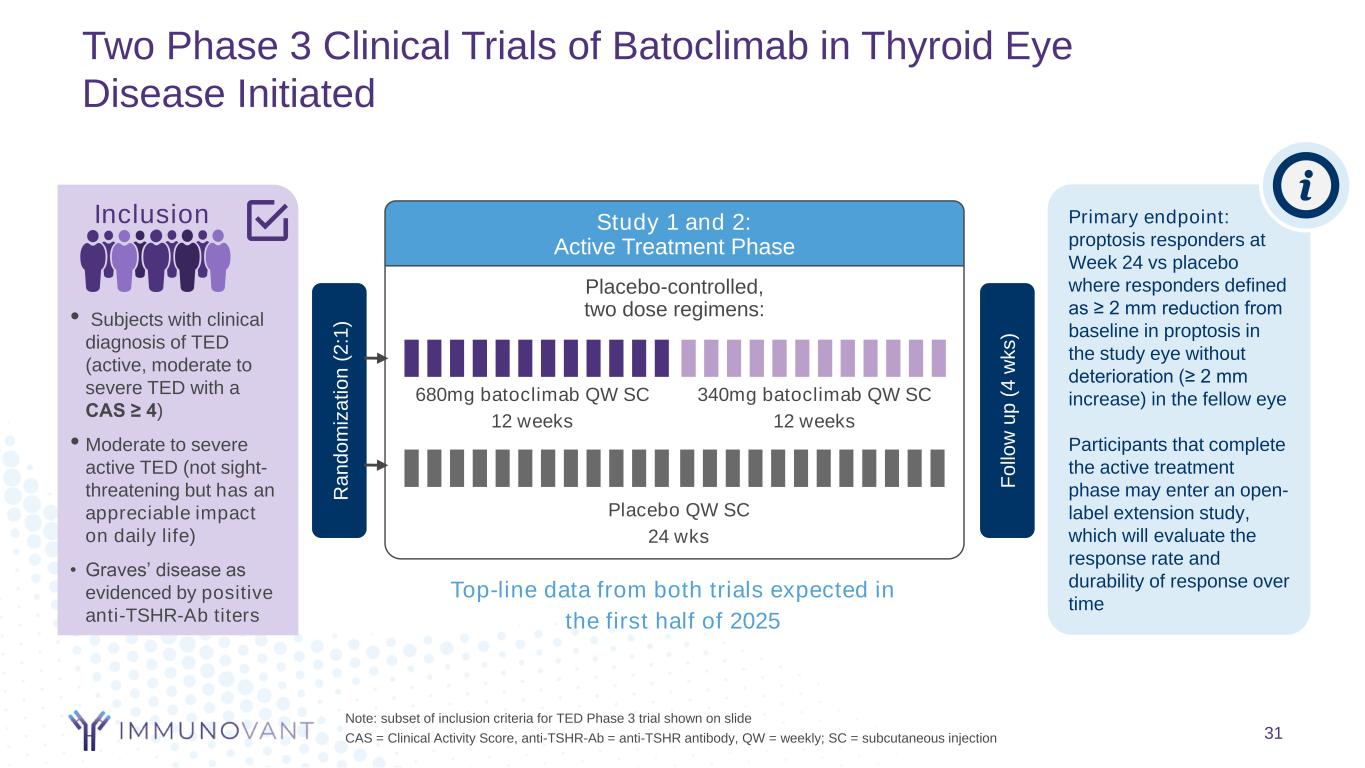
Two Phase 3 Clinical Trials of Batoclimab in Thyroid Eye Disease Initiated 31 Note: subset of inclusion criteria for TED Phase 3 trial shown on slide CAS = Clinical Activity Score, anti-TSHR-Ab = anti-TSHR antibody, QW = weekly; SC = subcutaneous injection Placebo-controlled, two dose regimens: Study 1 and 2: Active Treatment Phase Placebo QW SC 24 wks 340mg batoclimab QW SC 12 weeks 680mg batoclimab QW SC 12 weeks Primary endpoint: proptosis responders at Week 24 vs placebo where responders defined as ≥ 2 mm reduction from baseline in proptosis in the study eye without deterioration (≥ 2 mm increase) in the fellow eye Participants that complete the active treatment phase may enter an open- label extension study, which will evaluate the response rate and durability of response over time R a n d o m iz a ti o n ( 2 :1 ) Top-line data from both trials expected in the first half of 2025 F o llo w u p ( 4 w k s ) Inclusion • Subjects with clinical diagnosis of TED (active, moderate to severe TED with a CAS ≥ 4) • Moderate to severe active TED (not sight- threatening but has an appreciable impact on daily life) • Graves’ disease as evidenced by positive anti-TSHR-Ab titers
Chronic Inflammatory Demyelinating Polyneuropathy
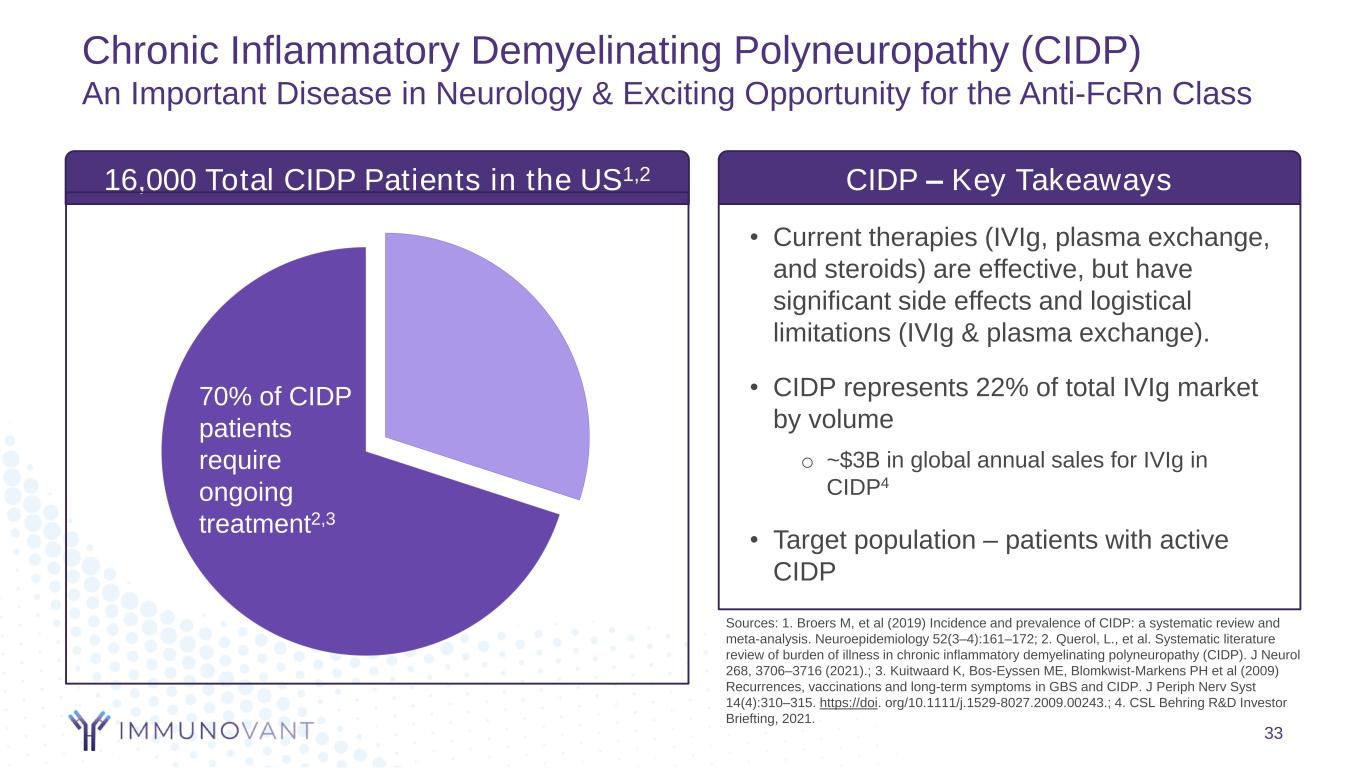
33 Sources: 1. Broers M, et al (2019) Incidence and prevalence of CIDP: a systematic review and meta-analysis. Neuroepidemiology 52(3–4):161–172; 2. Querol, L., et al. Systematic literature review of burden of illness in chronic inflammatory demyelinating polyneuropathy (CIDP). J Neurol 268, 3706–3716 (2021).; 3. Kuitwaard K, Bos-Eyssen ME, Blomkwist-Markens PH et al (2009) Recurrences, vaccinations and long-term symptoms in GBS and CIDP. J Periph Nerv Syst 14(4):310–315. https://doi. org/10.1111/j.1529-8027.2009.00243.; 4. CSL Behring R&D Investor Briefting, 2021. 70% of CIDP patients require ongoing treatment2,3 • Current therapies (IVIg, plasma exchange, and steroids) are effective, but have significant side effects and logistical limitations (IVIg & plasma exchange). • CIDP represents 22% of total IVIg market by volume o ~$3B in global annual sales for IVIg in CIDP4 • Target population – patients with active CIDP CIDP – Key Takeaways 16,000 Total CIDP Patients in the US1,2 70% of CIDP patients require ongoing treatment2,3 Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) An Important Disease in Neurology & Exciting Opportunity for the Anti-FcRn Class
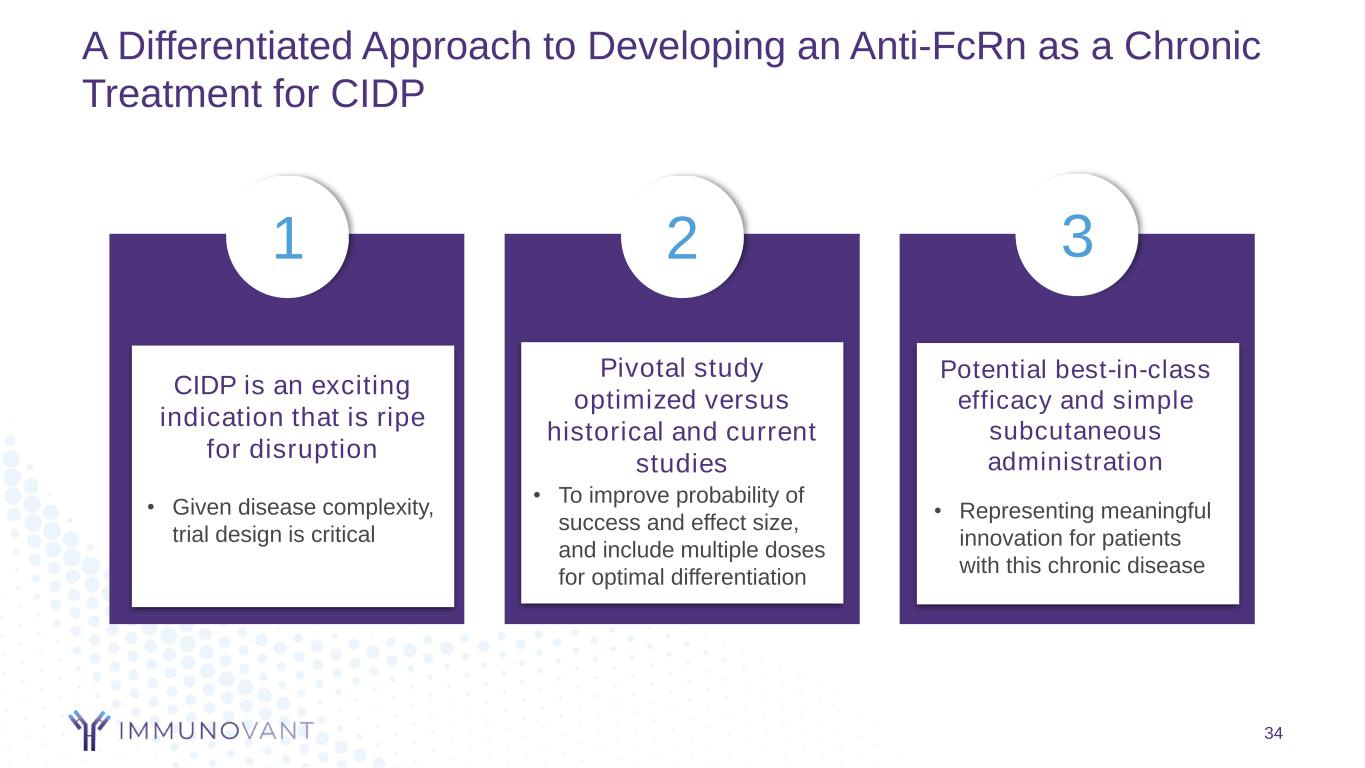
A Differentiated Approach to Developing an Anti-FcRn as a Chronic Treatment for CIDP 34 CIDP is an exciting indication that is ripe for disruption Pivotal study optimized versus historical and current studies Potential best-in-class efficacy and simple subcutaneous administration • Representing meaningful innovation for patients with this chronic disease • To improve probability of success and effect size, and include multiple doses for optimal differentiation • Given disease complexity, trial design is critical 1 2 3
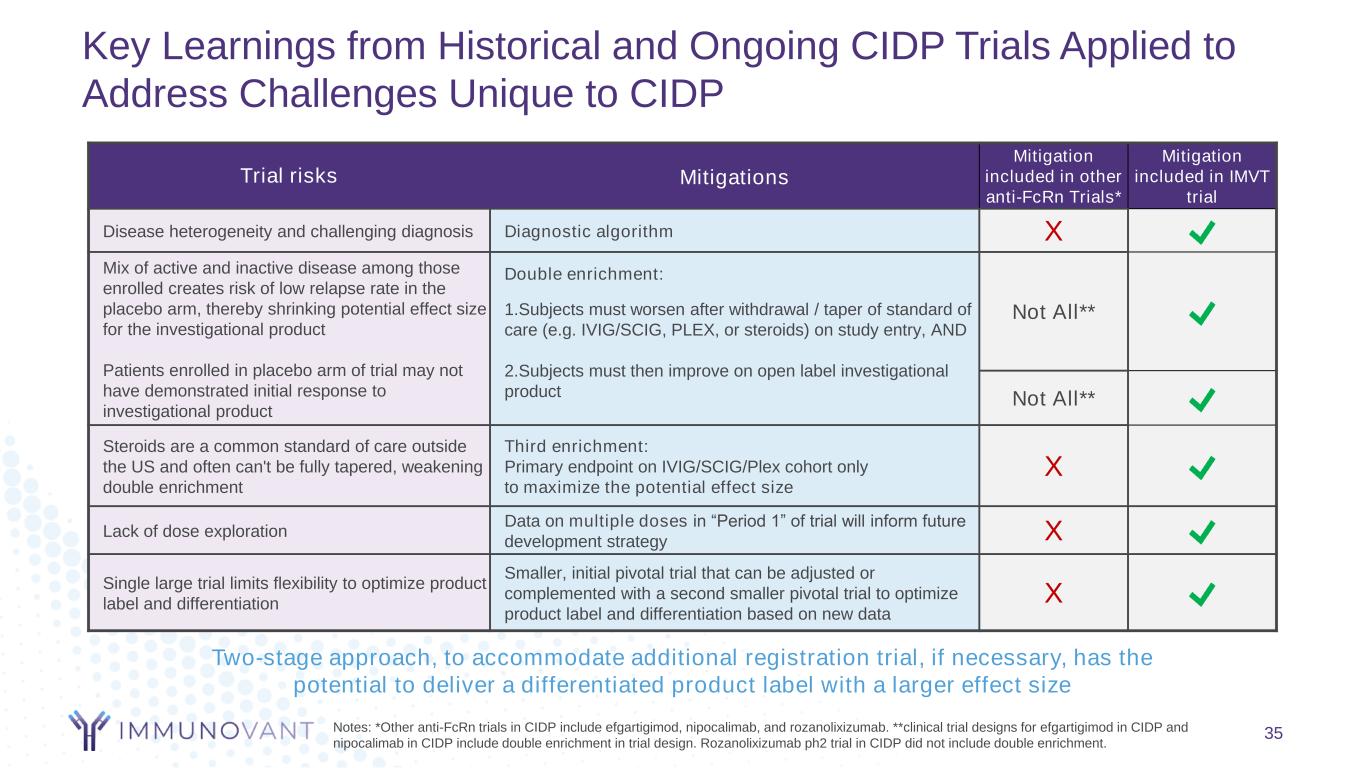
Key Learnings from Historical and Ongoing CIDP Trials Applied to Address Challenges Unique to CIDP 35 Trial risks Mitigations Mitigation included in other anti-FcRn Trials* Mitigation included in IMVT trial Disease heterogeneity and challenging diagnosis Diagnostic algorithm X Mix of active and inactive disease among those enrolled creates risk of low relapse rate in the placebo arm, thereby shrinking potential effect size for the investigational product Patients enrolled in placebo arm of trial may not have demonstrated initial response to investigational product Double enrichment: Not All**1.Subjects must worsen after withdrawal / taper of standard of care (e.g. IVIG/SCIG, PLEX, or steroids) on study entry, AND 2.Subjects must then improve on open label investigational product Not All** Steroids are a common standard of care outside the US and often can't be fully tapered, weakening double enrichment Third enrichment: Primary endpoint on IVIG/SCIG/Plex cohort only to maximize the potential effect size X Lack of dose exploration Data on multiple doses in “Period 1” of trial will inform future development strategy X Single large trial limits flexibility to optimize product label and differentiation Smaller, initial pivotal trial that can be adjusted or complemented with a second smaller pivotal trial to optimize product label and differentiation based on new data X Notes: *Other anti-FcRn trials in CIDP include efgartigimod, nipocalimab, and rozanolixizumab. **clinical trial designs for efgartigimod in CIDP and nipocalimab in CIDP include double enrichment in trial design. Rozanolixizumab ph2 trial in CIDP did not include double enrichment. Two-stage approach, to accommodate additional registration trial, if necessary, has the potential to deliver a differentiated product label with a larger effect size
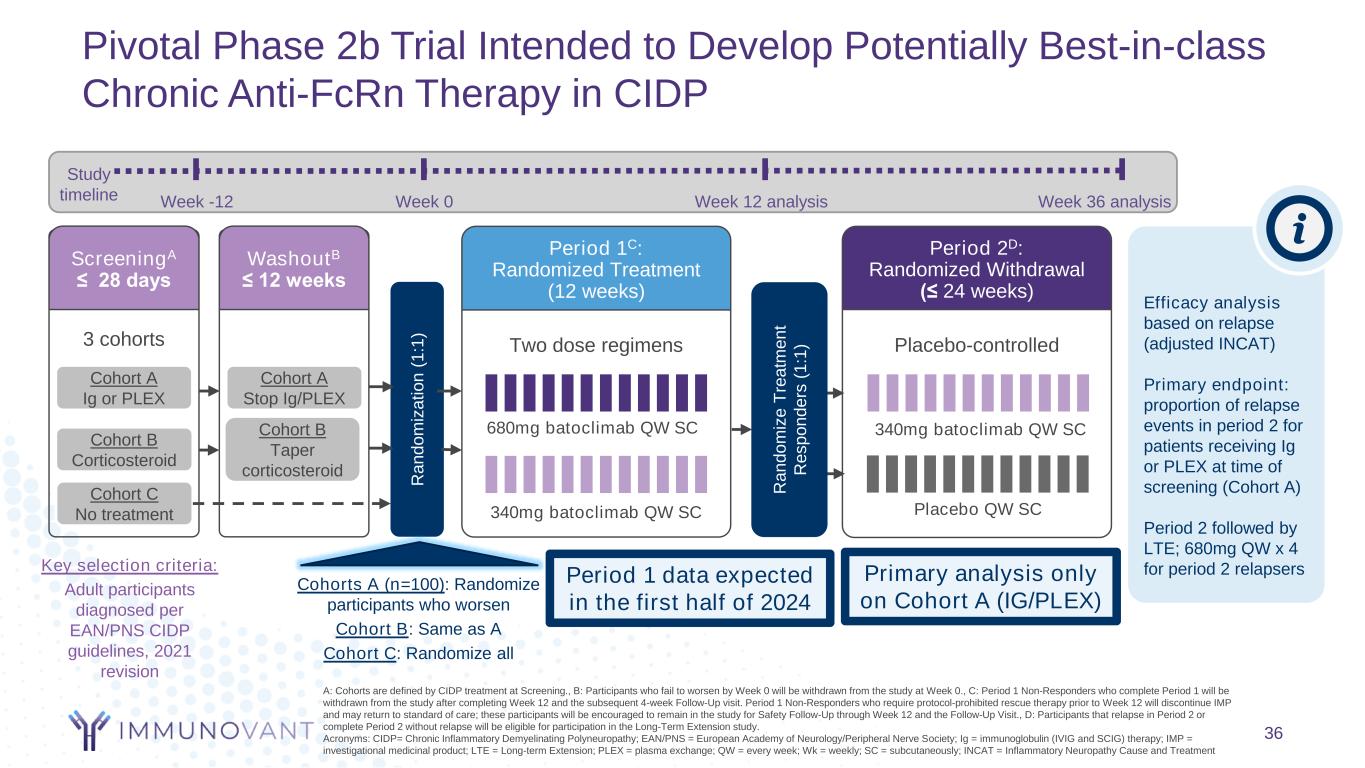
Pivotal Phase 2b Trial Intended to Develop Potentially Best-in-class Chronic Anti-FcRn Therapy in CIDP A: Cohorts are defined by CIDP treatment at Screening., B: Participants who fail to worsen by Week 0 will be withdrawn from the study at Week 0., C: Period 1 Non-Responders who complete Period 1 will be withdrawn from the study after completing Week 12 and the subsequent 4-week Follow-Up visit. Period 1 Non-Responders who require protocol-prohibited rescue therapy prior to Week 12 will discontinue IMP and may return to standard of care; these participants will be encouraged to remain in the study for Safety Follow-Up through Week 12 and the Follow-Up Visit., D: Participants that relapse in Period 2 or complete Period 2 without relapse will be eligible for participation in the Long-Term Extension study. Acronyms: CIDP= Chronic Inflammatory Demyelinating Polyneuropathy; EAN/PNS = European Academy of Neurology/Peripheral Nerve Society; Ig = immunoglobulin (IVIG and SCIG) therapy; IMP = investigational medicinal product; LTE = Long-term Extension; PLEX = plasma exchange; QW = every week; Wk = weekly; SC = subcutaneously; INCAT = Inflammatory Neuropathy Cause and Treatment Two dose regimens Period 1C: Randomized Treatment (12 weeks) Placebo-controlled Period 2D: Randomized Withdrawal (≤ 24 weeks) Placebo QW SC340mg batoclimab QW SC 680mg batoclimab QW SC 340mg batoclimab QW SC Efficacy analysis based on relapse (adjusted INCAT) Primary endpoint: proportion of relapse events in period 2 for patients receiving Ig or PLEX at time of screening (Cohort A) Period 2 followed by LTE; 680mg QW x 4 for period 2 relapsers R a n d o m iz e T re a tm e n t R e s p o n d e rs ( 1 :1 )3 cohorts ScreeningA ≤ 28 days Cohort A Ig or PLEX Cohort B Corticosteroid Cohort C No treatment R a n d o m iz a ti o n ( 1 :1 ) Cohort A Stop Ig/PLEX Cohort B Taper corticosteroid Key selection criteria: Adult participants diagnosed per EAN/PNS CIDP guidelines, 2021 revision Week -12 Week 0 Week 12 analysis Week 36 analysis Study timeline Primary analysis only on Cohort A (IG/PLEX) WashoutB ≤ 12 weeks 36 Cohorts A (n=100): Randomize participants who worsen Cohort B: Same as A Cohort C: Randomize all Period 1 data expected in the first half of 2024

Graves’ Disease
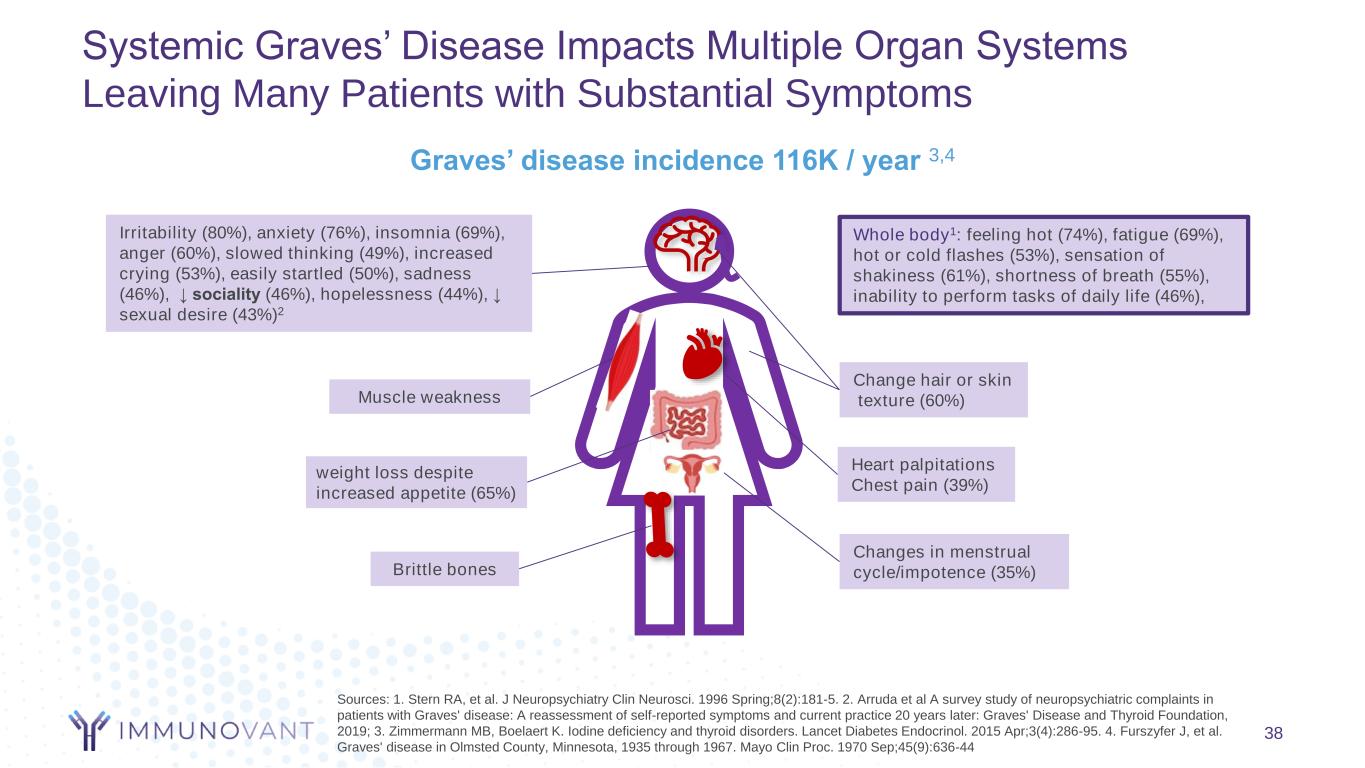
Systemic Graves’ Disease Impacts Multiple Organ Systems Leaving Many Patients with Substantial Symptoms 38 Sources: 1. Stern RA, et al. J Neuropsychiatry Clin Neurosci. 1996 Spring;8(2):181-5. 2. Arruda et al A survey study of neuropsychiatric complaints in patients with Graves' disease: A reassessment of self-reported symptoms and current practice 20 years later: Graves' Disease and Thyroid Foundation, 2019; 3. Zimmermann MB, Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015 Apr;3(4):286-95. 4. Furszyfer J, et al. Graves' disease in Olmsted County, Minnesota, 1935 through 1967. Mayo Clin Proc. 1970 Sep;45(9):636-44 Irritability (80%), anxiety (76%), insomnia (69%), anger (60%), slowed thinking (49%), increased crying (53%), easily startled (50%), sadness (46%), ↓ sociality (46%), hopelessness (44%), ↓ sexual desire (43%)2 weight loss despite increased appetite (65%) Whole body1: feeling hot (74%), fatigue (69%), hot or cold flashes (53%), sensation of shakiness (61%), shortness of breath (55%), inability to perform tasks of daily life (46%), Brittle bones Muscle weakness Change hair or skin texture (60%) Heart palpitations Chest pain (39%) Changes in menstrual cycle/impotence (35%) Graves’ disease incidence 116K / year 3,4
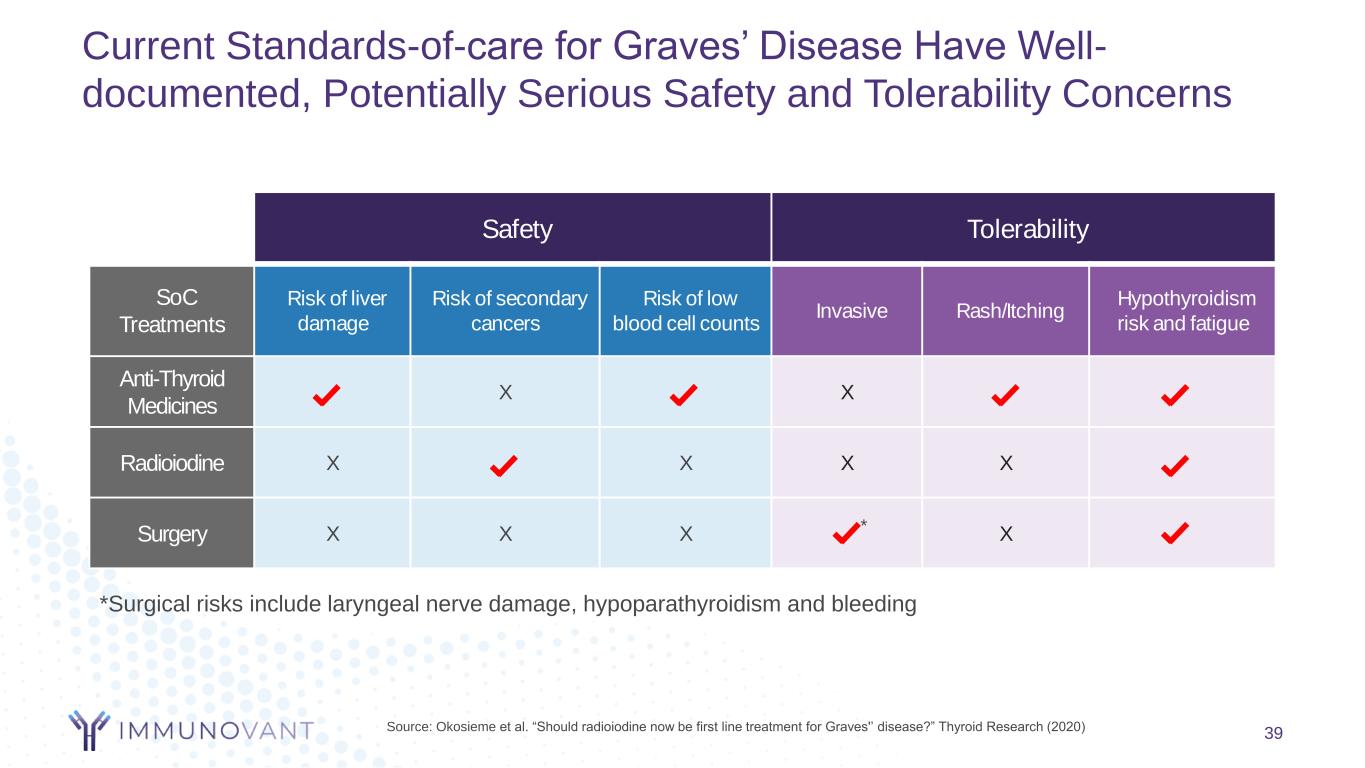
Current Standards-of-care for Graves’ Disease Have Well- documented, Potentially Serious Safety and Tolerability Concerns 39 Safety Tolerability SoC Treatments Risk of liver damage Risk of secondary cancers Risk of low blood cell counts Invasive Rash/Itching Hypothyroidism risk and fatigue Anti-Thyroid Medicines X X Radioiodine X X X X Surgery X X X * X Source: Okosieme et al. “Should radioiodine now be first line treatment for Graves'’ disease?” Thyroid Research (2020) *Surgical risks include laryngeal nerve damage, hypoparathyroidism and bleeding
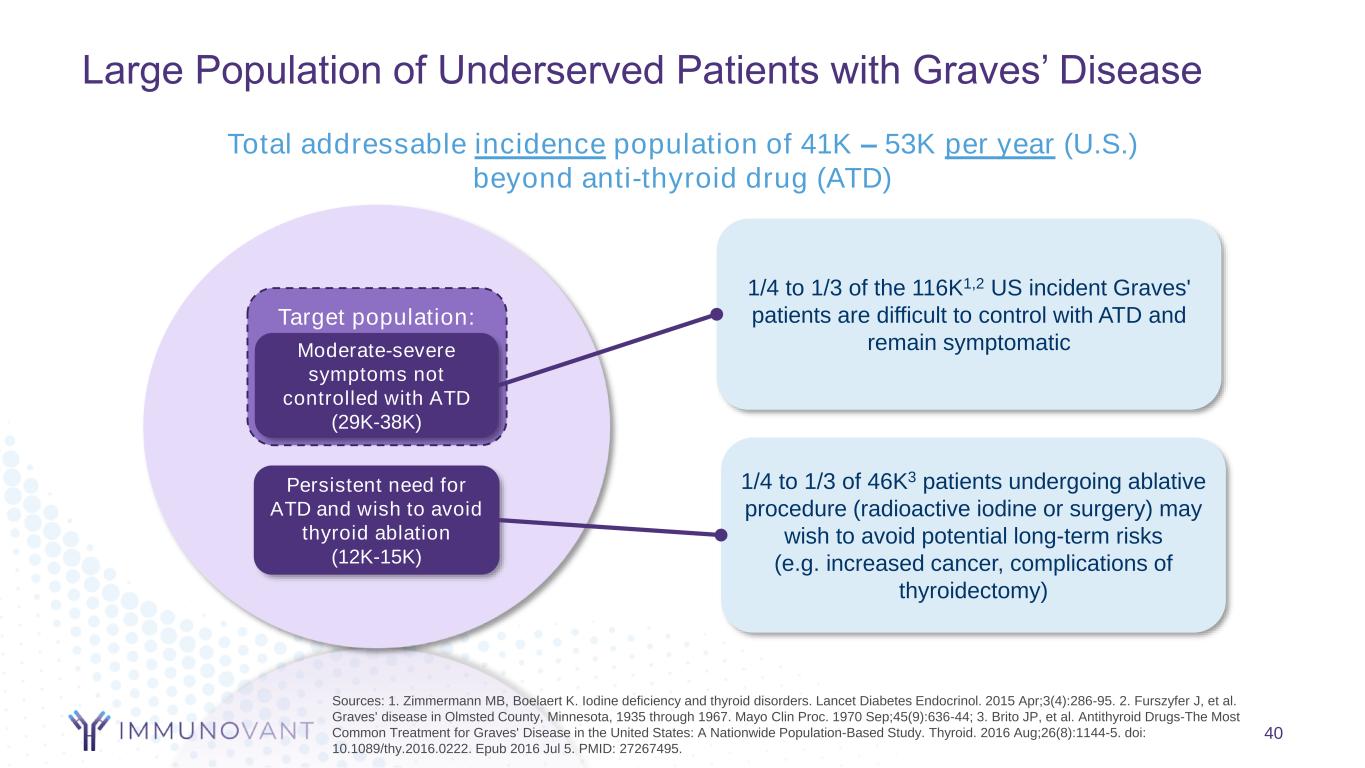
Target population: 40 Total addressable incidence population of 41K – 53K per year (U.S.) beyond anti-thyroid drug (ATD) Persistent need for ATD and wish to avoid thyroid ablation (12K-15K) Moderate-severe symptoms not controlled with ATD (29K-38K) Sources: 1. Zimmermann MB, Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015 Apr;3(4):286-95. 2. Furszyfer J, et al. Graves' disease in Olmsted County, Minnesota, 1935 through 1967. Mayo Clin Proc. 1970 Sep;45(9):636-44; 3. Brito JP, et al. Antithyroid Drugs-The Most Common Treatment for Graves' Disease in the United States: A Nationwide Population-Based Study. Thyroid. 2016 Aug;26(8):1144-5. doi: 10.1089/thy.2016.0222. Epub 2016 Jul 5. PMID: 27267495. 1/4 to 1/3 of the 116K1,2 US incident Graves' patients are difficult to control with ATD and remain symptomatic 1/4 to 1/3 of 46K3 patients undergoing ablative procedure (radioactive iodine or surgery) may wish to avoid potential long-term risks (e.g. increased cancer, complications of thyroidectomy) Large Population of Underserved Patients with Graves’ Disease
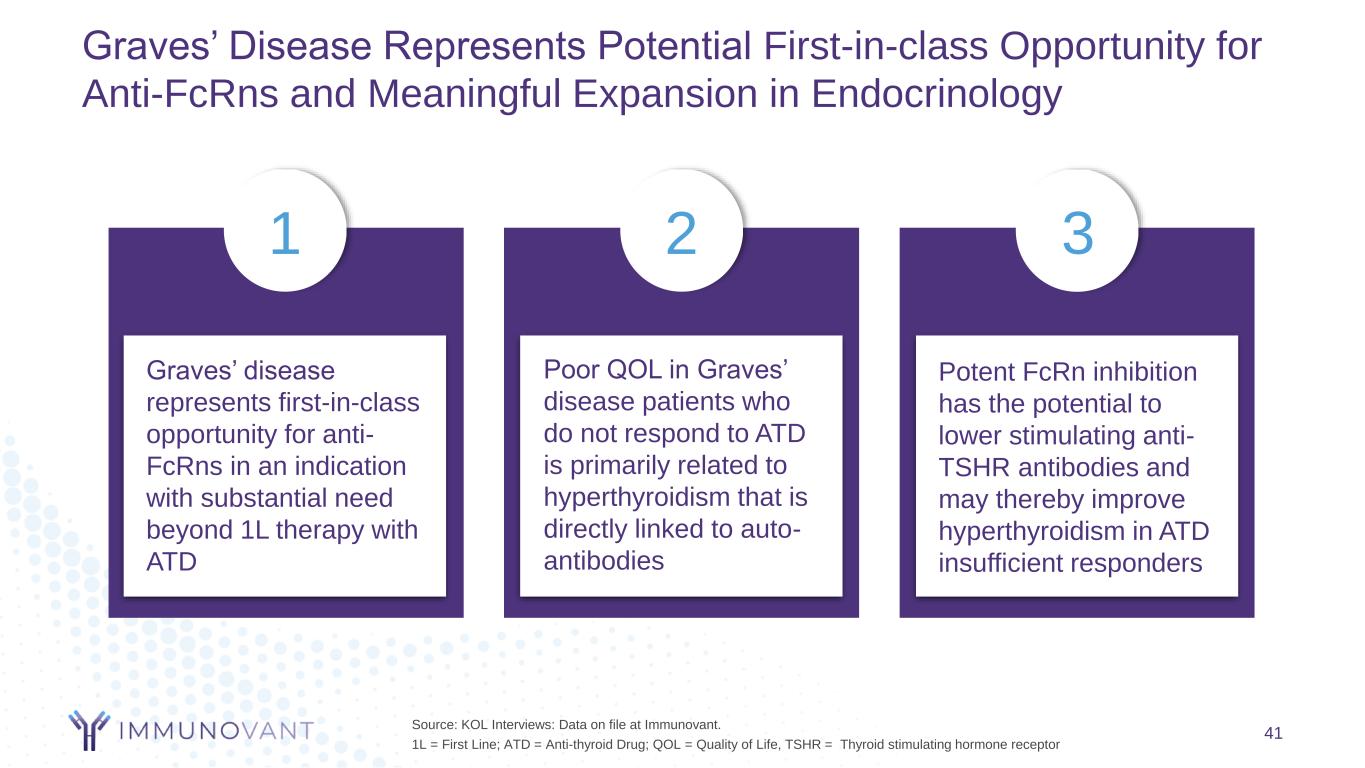
Graves’ Disease Represents Potential First-in-class Opportunity for Anti-FcRns and Meaningful Expansion in Endocrinology 41 Graves’ disease represents first-in-class opportunity for anti- FcRns in an indication with substantial need beyond 1L therapy with ATD Poor QOL in Graves’ disease patients who do not respond to ATD is primarily related to hyperthyroidism that is directly linked to auto- antibodies Potent FcRn inhibition has the potential to lower stimulating anti- TSHR antibodies and may thereby improve hyperthyroidism in ATD insufficient responders Source: KOL Interviews: Data on file at Immunovant. 1L = First Line; ATD = Anti-thyroid Drug; QOL = Quality of Life, TSHR = Thyroid stimulating hormone receptor 1 2 3
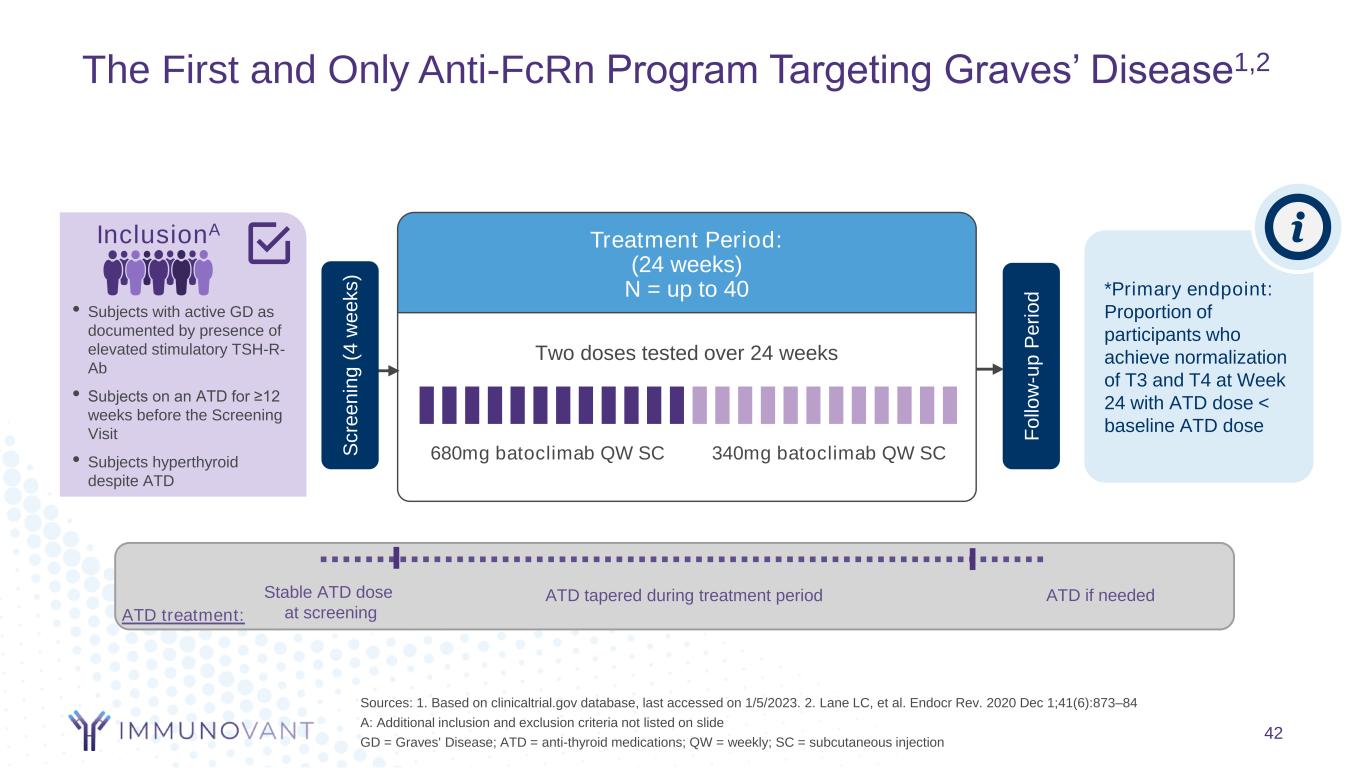
The First and Only Anti-FcRn Program Targeting Graves’ Disease1,2 42 Sources: 1. Based on clinicaltrial.gov database, last accessed on 1/5/2023. 2. Lane LC, et al. Endocr Rev. 2020 Dec 1;41(6):873–84 A: Additional inclusion and exclusion criteria not listed on slide GD = Graves' Disease; ATD = anti-thyroid medications; QW = weekly; SC = subcutaneous injection Two doses tested over 24 weeks Treatment Period: (24 weeks) N = up to 40 340mg batoclimab QW SC680mg batoclimab QW SC *Primary endpoint: Proportion of participants who achieve normalization of T3 and T4 at Week 24 with ATD dose < baseline ATD doseF o llo w -u p P e ri o d InclusionA • Subjects with active GD as documented by presence of elevated stimulatory TSH-R- Ab • Subjects on an ATD for ≥12 weeks before the Screening Visit • Subjects hyperthyroid despite ATD S c re e n in g ( 4 w e e k s ) ATD treatment: Stable ATD dose at screening ATD tapered during treatment period ATD if needed
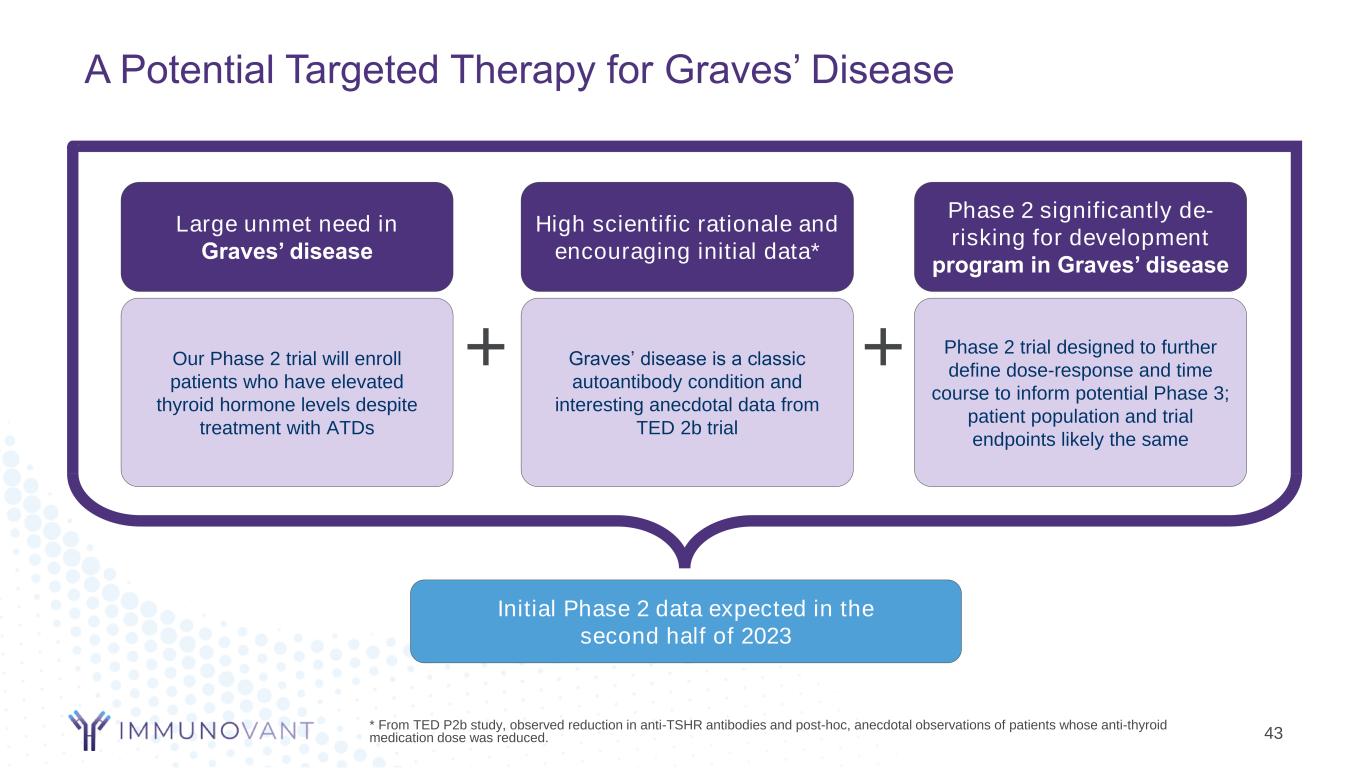
A Potential Targeted Therapy for Graves’ Disease * From TED P2b study, observed reduction in anti-TSHR antibodies and post-hoc, anecdotal observations of patients whose anti-thyroid medication dose was reduced. Initial Phase 2 data expected in the second half of 2023 Large unmet need in Graves’ disease Our Phase 2 trial will enroll patients who have elevated thyroid hormone levels despite treatment with ATDs High scientific rationale and encouraging initial data* Graves’ disease is a classic autoantibody condition and interesting anecdotal data from TED 2b trial Phase 2 significantly de- risking for development program in Graves’ disease Phase 2 trial designed to further define dose-response and time course to inform potential Phase 3; patient population and trial endpoints likely the same + + 43

Warm Autoimmune Hemolytic Anemia
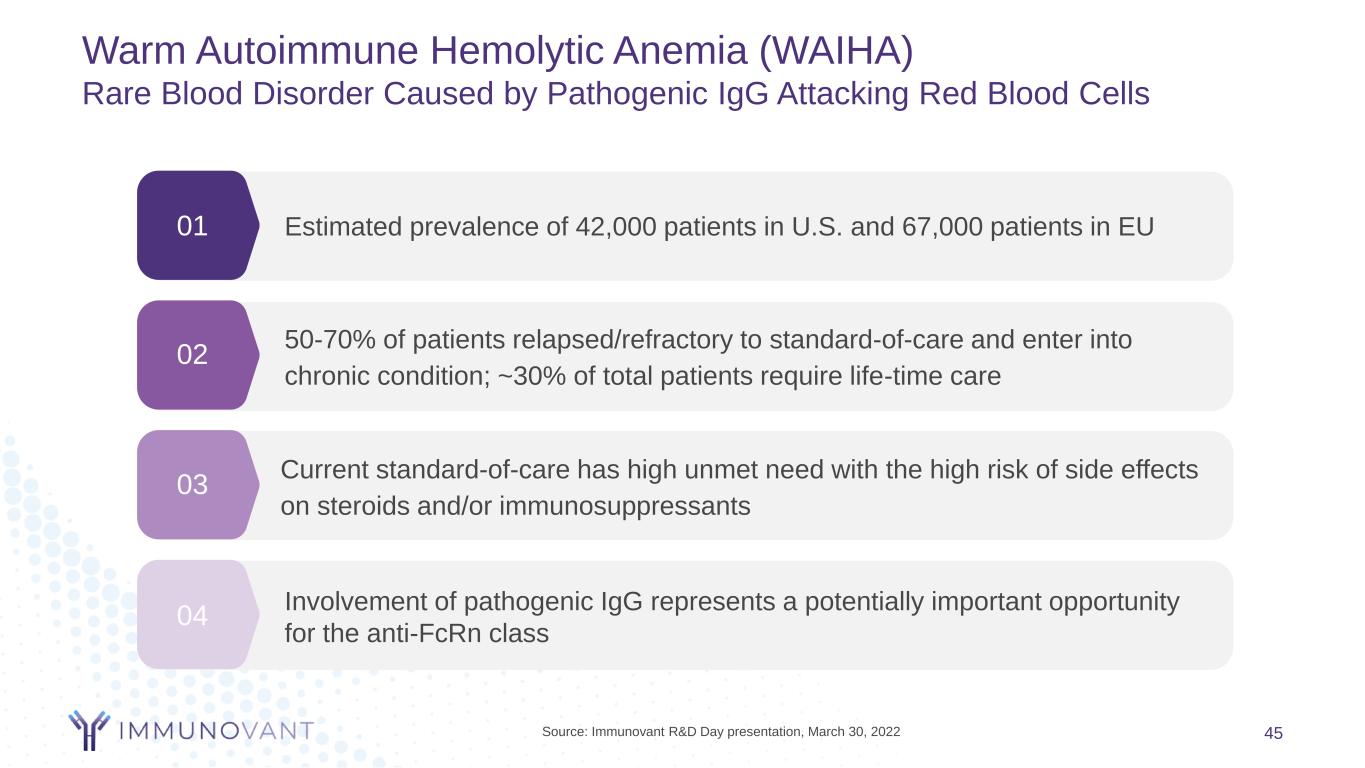
01 02 03 Current standard-of-care has high unmet need with the high risk of side effects on steroids and/or immunosuppressants 50-70% of patients relapsed/refractory to standard-of-care and enter into chronic condition; ~30% of total patients require life-time care Estimated prevalence of 42,000 patients in U.S. and 67,000 patients in EU 04 Involvement of pathogenic IgG represents a potentially important opportunity for the anti-FcRn class Warm Autoimmune Hemolytic Anemia (WAIHA) Rare Blood Disorder Caused by Pathogenic IgG Attacking Red Blood Cells 45Source: Immunovant R&D Day presentation, March 30, 2022
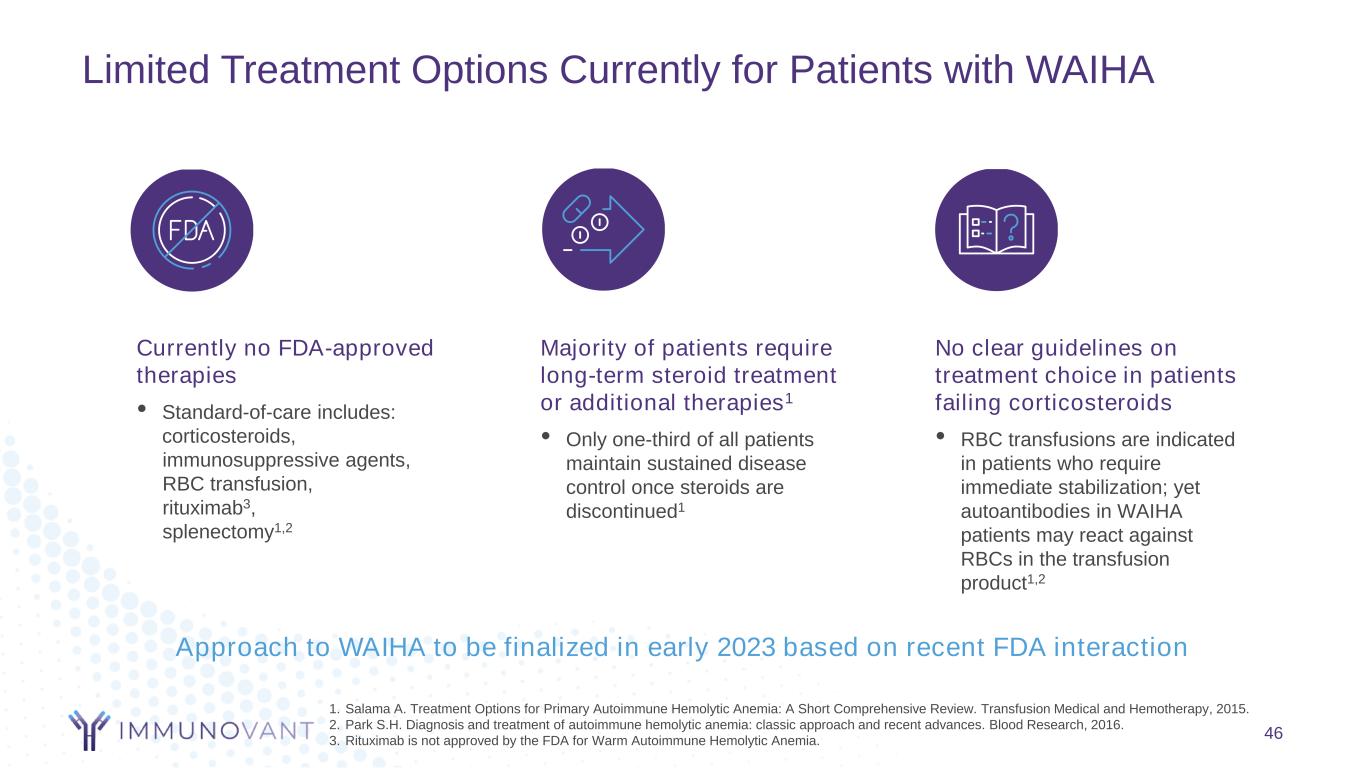
Limited Treatment Options Currently for Patients with WAIHA 46 Majority of patients require long-term steroid treatment or additional therapies1 • Only one-third of all patients maintain sustained disease control once steroids are discontinued1 Currently no FDA-approved therapies • Standard-of-care includes: corticosteroids, immunosuppressive agents, RBC transfusion, rituximab3, splenectomy1,2 No clear guidelines on treatment choice in patients failing corticosteroids • RBC transfusions are indicated in patients who require immediate stabilization; yet autoantibodies in WAIHA patients may react against RBCs in the transfusion product1,2 1. Salama A. Treatment Options for Primary Autoimmune Hemolytic Anemia: A Short Comprehensive Review. Transfusion Medical and Hemotherapy, 2015. 2. Park S.H. Diagnosis and treatment of autoimmune hemolytic anemia: classic approach and recent advances. Blood Research, 2016. 3. Rituximab is not approved by the FDA for Warm Autoimmune Hemolytic Anemia. Approach to WAIHA to be finalized in early 2023 based on recent FDA interaction

Building a Leading Anti-FcRn Franchise
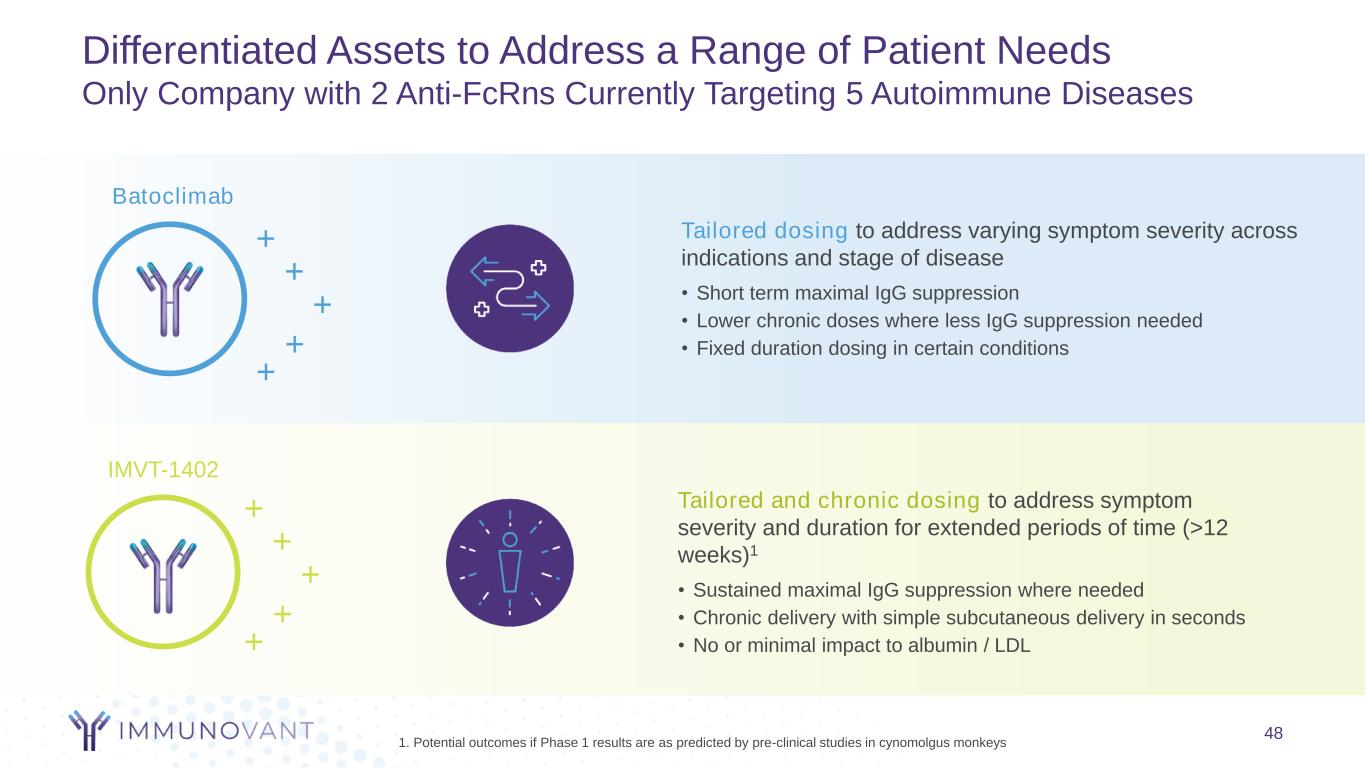
Differentiated Assets to Address a Range of Patient Needs Only Company with 2 Anti-FcRns Currently Targeting 5 Autoimmune Diseases 48 Tailored dosing to address varying symptom severity across indications and stage of disease • Short term maximal IgG suppression • Lower chronic doses where less IgG suppression needed • Fixed duration dosing in certain conditions Tailored and chronic dosing to address symptom severity and duration for extended periods of time (>12 weeks)1 • Sustained maximal IgG suppression where needed • Chronic delivery with simple subcutaneous delivery in seconds • No or minimal impact to albumin / LDL Batoclimab + + + + + IMVT-1402 + + + + + 1. Potential outcomes if Phase 1 results are as predicted by pre-clinical studies in cynomolgus monkeys
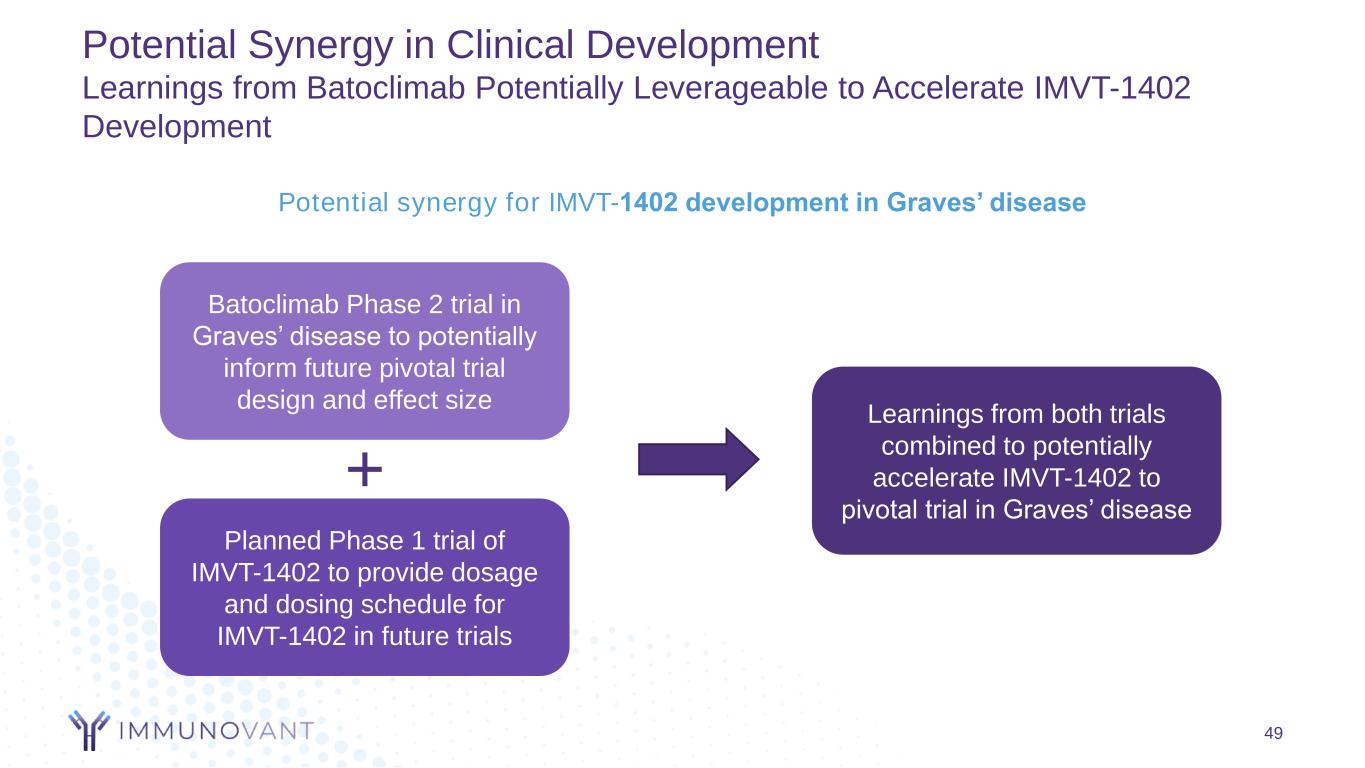
49 Potential Synergy in Clinical Development Learnings from Batoclimab Potentially Leverageable to Accelerate IMVT-1402 Development Potential synergy for IMVT-1402 development in Graves’ disease Learnings from both trials combined to potentially accelerate IMVT-1402 to pivotal trial in Graves’ disease Batoclimab Phase 2 trial in Graves’ disease to potentially inform future pivotal trial design and effect size Planned Phase 1 trial of IMVT-1402 to provide dosage and dosing schedule for IMVT-1402 in future trials
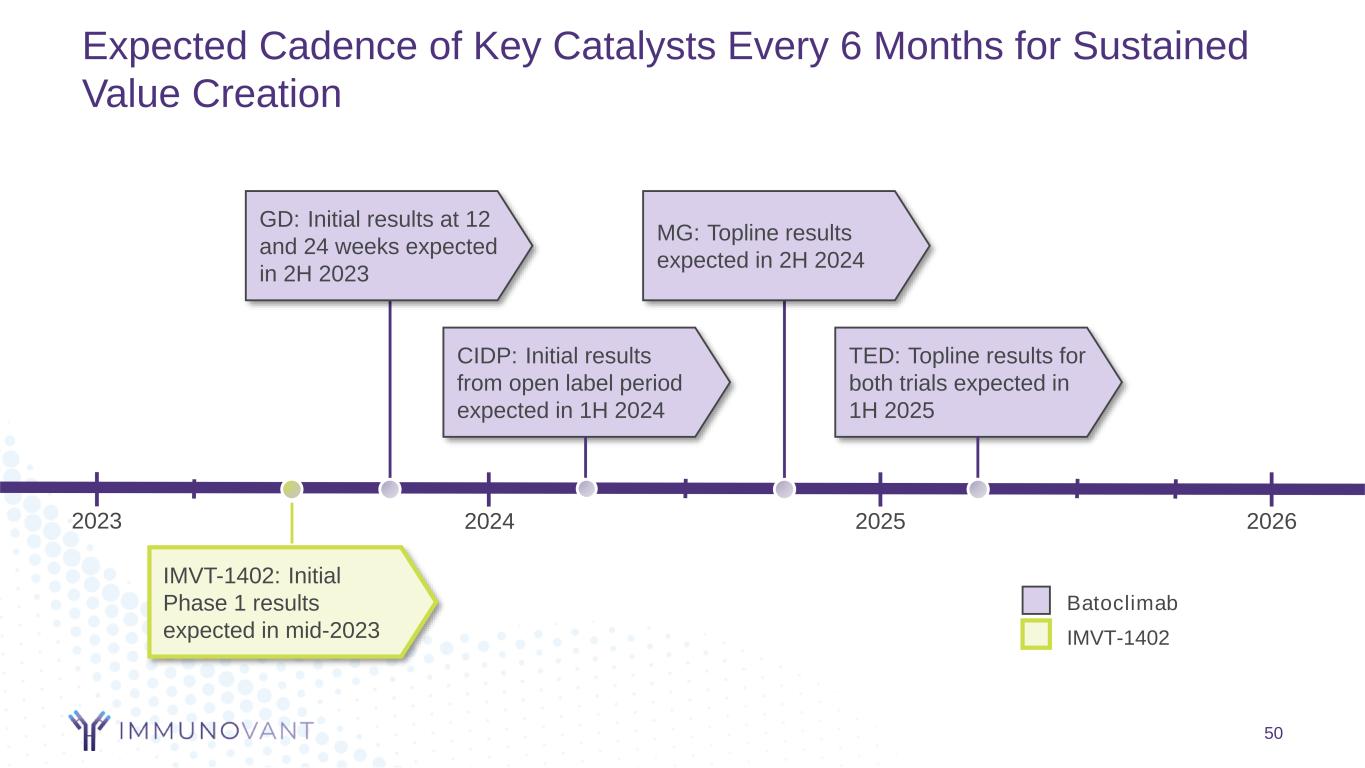
2023 Expected Cadence of Key Catalysts Every 6 Months for Sustained Value Creation 50 GD: Initial results at 12 and 24 weeks expected in 2H 2023 MG: Topline results expected in 2H 2024 2024 2025 TED: Topline results for both trials expected in 1H 2025 CIDP: Initial results from open label period expected in 1H 2024 2026 Batoclimab IMVT-1402 IMVT-1402: Initial Phase 1 results expected in mid-2023
51 Potentially first to develop subcutaneous anti-FcRn that can be administered in seconds Complementary anti-FcRns potentially enable accelerated development pathways Cultivating broad network of experts to optimize multi- indication development plan Love Trailblazing Bolder, Faster All Voices Our Vision: Normal Lives for People with Autoimmune Disease

Thank you