
Immunovant Announces Plans to Study Batoclimab in Two New Indications Committed to enabling normal lives for people with autoimmune disease September 7, 2022 EExhibit 99.1

Forward-looking statements 2 This presentation contains forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. The use of words such as "can," “may,” “might,” “will,” “would,” “should,” “expect,” “believe,” “estimate,” “design,” “plan,” "intend," and other similar expressions are intended to identify forward-looking statements. Such forward looking statements include Immunovant's plan to initiate a Phase 2b clinical trial for batoclimab in Chronic Inflammatory Demyelinating Polyneuropathy in the second half of calendar year 2022 with initial results from open-label period 1 expected in the first half of calendar year 2024; Immunovant's plan to initiate a Phase 2 clinical trial for batoclimab in Graves' Disease in early 2023 with initial results expected in the second half of calendar year 2023; Immunovant’s plan to report topline data from its Phase 3 trial for batoclimab in Myasthenia Gravis in the second half of calendar year 2024; Immunovant's plan to initiate two Phase 3 clinical trials for batoclimab in Thyroid Eye Disease in the second half of calendar year 2022 with expected topline data readouts in the first half of calendar year 2025; Immunovant’s plan to finalize its trial design in Warm Autoimmune Hemolytic Anemia following expected interactions with regulators later in calendar year 2022; Immunovant's plan to develop batoclimab across a broad range of autoimmune indications; expectations with respect to the safety and monitoring plan and size of the safety database for these planned clinical trials; the timing of discussions with regulatory agencies; the size and growth of the potential markets for Immunovant's product candidates and indication selections; Immunovant’s plan to explore in subsequent study periods follow-on treatment with alternative dosing regimens; Immunovant’s expectations regarding patient enrollment, timing, the design and results of clinical trials of its product candidates and indication selections; Immunovant's beliefs regarding its cash runway, and the potential benefits of batoclimab’s unique product attributes. All forward-looking statements are based on estimates and assumptions by Immunovant’s management that, although Immunovant believes to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that Immunovant expected. Such risks and uncertainties include, among others: initial results or other preliminary analyses or results of early clinical trials may not be predictive final trial results or of the results of later clinical trials; the timing and availability of data from clinical trials; the timing of discussions with regulatory agencies, as well as regulatory submissions and potential approvals; the continued development of Immunovant’s product candidate, including the timing of the commencement of additional clinical trials and resumption of current trials; Immunovant’s scientific approach, clinical trial design, indication selection and general development progress; future clinical trials may not confirm any safety, potency or other product characteristics described or assumed in this presentation; any product candidate that Immunovant develops may not progress through clinical development or receive required regulatory approvals within expected timelines or at all; Immunovant’s product candidate may not be beneficial to patients, or even if approved by regulatory authorities, successfully commercialized; the potential impact of the ongoing COVID-19 pandemic, geopolitical tensions, and adverse macroeconomic conditions on Immunovant’s clinical development plans and timelines; Immunovant’s business is heavily dependent on the successful development, regulatory approval and commercialization of its sole product candidate, batoclimab; Immunovant is at an early stage in development of batoclimab; and Immunovant will require additional capital to fund its operations and advance batoclimab through clinical development. These and other risks and uncertainties are more fully described in Immunovant’s periodic and other reports filed with the Securities and Exchange Commission (SEC), including in the section titled “Risk Factors” in Immunovant’s most recent Annual Report on Form 10-K, its Form 10-Q filed with the SEC on August 5, 2022, and Immunovant’s subsequent filings with the SEC. Any forward-looking statement speaks only as of the date on which it was made. Immunovant undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. All trademarks, trade names, service marks, and copyrights appearing in this presentation are the property of their respective owners. Dates used in this presentation refer to the applicable calendar year unless otherwise noted.

Q&A housekeeping 3 You may submit questions through the Q&A function at the bottom of the webcast player. Please see the image here for reference. For Investor Audiences Only

Today’s agenda Immunovant and batoclimab Introduction • Pete Salzmann, MD, CEO Immunovant Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Differentiated development program to optimize effect size • Jonathan Katz1, MD, Director Neuromuscular Clinic, California Pacific Medical Center • Todd Levine2, Medical Director, Neurology, Honor Health Scottsdale, Arizona • Pete Salzmann, MD, CEO Immunovant Graves' Disease First in class development program in high unmet need population • George Kahaly3, MD, PhD, Johannes Gutenberg University Medical Center • Pete Salzmann, MD, CEO Immunovant Closing Summary of milestones and catalysts • Pete Salzmann, MD, CEO Immunovant Q&A 4 Financial Disclosures: 1. Consultant for Argenx, Griffols, MT Pharma, Biogen, Amylyx, Alexion, PTC Therapeutics, Calico, UCB; 2. Eledon, Speakers’ bureau for Griffols. Financial interests in CND Life Sciences and CRL. Consult for InCircle; 3. The Johannes Gutenberg University (JGU) Medical Center, Mainz, Germany (academic institution of George J Kahaly, MD, PhD) has received research-associated funding from the JGU Medical Faculty, AdvanceCor (Germany), Apitope (Belgium), Berlin-Chemie (Germany), Byondis (The Netherlands), GlycoEra (Switzerland), Horizon (USA), Immunovant (USA), ISAR (Germany), Mediomics (USA), Merck (Germany), Novartis (USA), Quidel (USA), River Vision (USA), and Roche (Switzerland). GJK consults for GlycoEra, Immunovant, ISAR, Mediomics, Merck, Novartis, Quidel, & VasaraGen (USA). For Investor Audiences Only
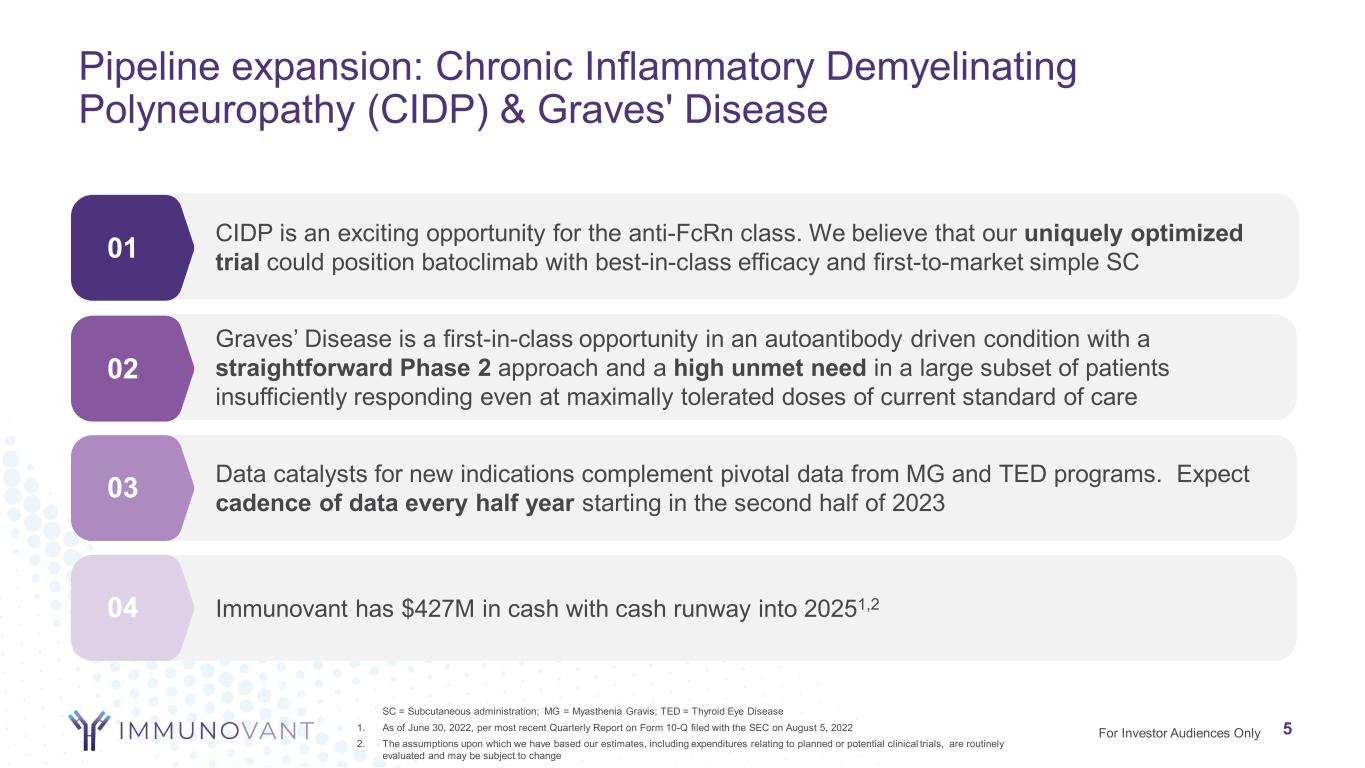
Pipeline expansion: Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) & Graves' Disease 5 01 02 03 SC = Subcutaneous administration; MG = Myasthenia Gravis; TED = Thyroid Eye Disease 1. As of June 30, 2022, per most recent Quarterly Report on Form 10-Q filed with the SEC on August 5, 2022 2. The assumptions upon which we have based our estimates, including expenditures relating to planned or potential clinical trials, are routinely evaluated and may be subject to change 5For Investor Audiences Only Data catalysts for new indications complement pivotal data from MG and TED programs. Expect cadence of data every half year starting in the second half of 2023 Graves’ Disease is a first-in-class opportunity in an autoantibody driven condition with a straightforward Phase 2 approach and a high unmet need in a large subset of patients insufficiently responding even at maximally tolerated doses of current standard of care CIDP is an exciting opportunity for the anti-FcRn class. We believe that our uniquely optimized trial could position batoclimab with best-in-class efficacy and first-to-market simple SC 04 Immunovant has $427M in cash with cash runway into 20251,2

Pete Salzmann, MD Chief Executive Officer Immunovant and Batoclimab
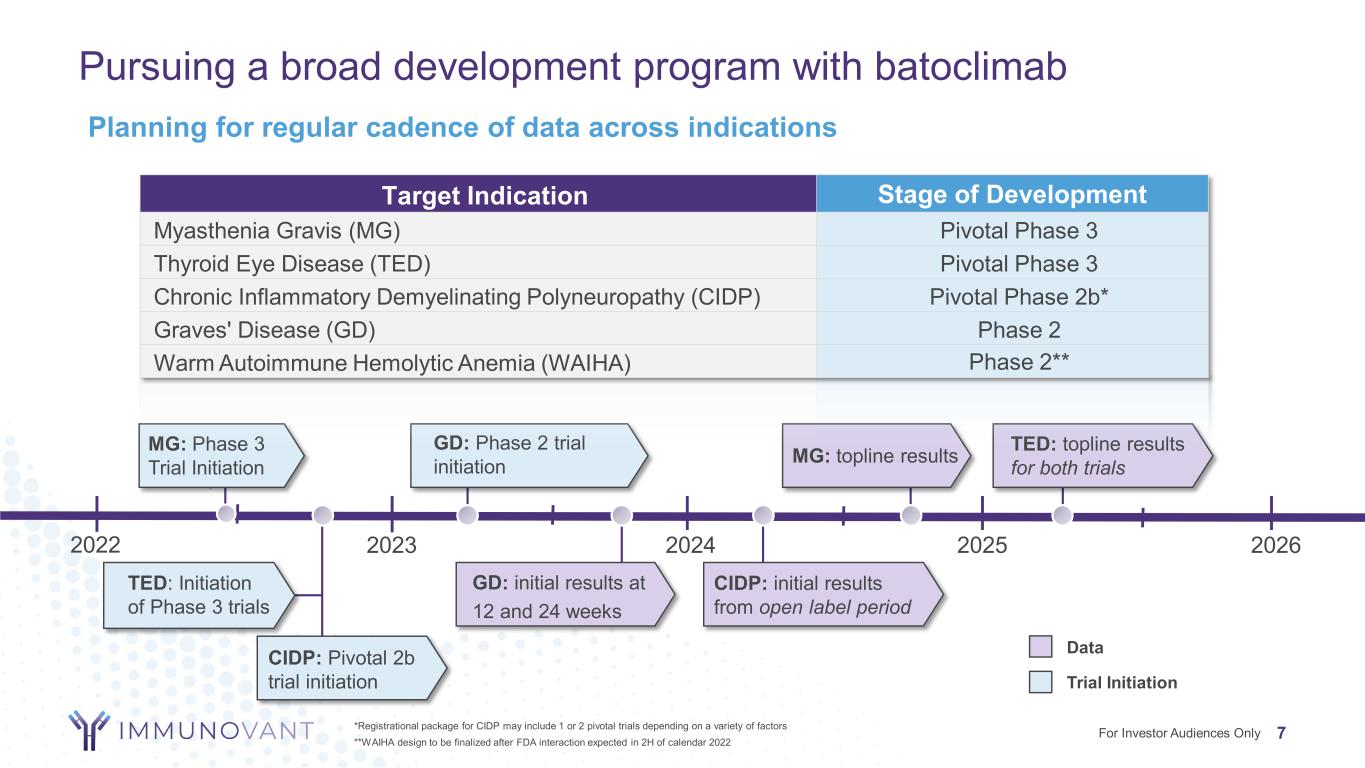
Pursuing a broad development program with batoclimab 7*Registrational package for CIDP may include 1 or 2 pivotal trials depending on a variety of factors **WAIHA design to be finalized after FDA interaction expected in 2H of calendar 2022 For Investor Audiences Only GD: Phase 2 trial initiation GD: initial results at 12 and 24 weeks MG: topline results 2023 2024 2025 MG: Phase 3 Trial Initiation TED: Initiation of Phase 3 trials TED: topline results for both trials CIDP: initial results from open label period CIDP: Pivotal 2b trial initiation Target Indication Stage of Development Myasthenia Gravis (MG) Pivotal Phase 3 Thyroid Eye Disease (TED) Pivotal Phase 3 Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Pivotal Phase 2b* Graves' Disease (GD) Phase 2 Warm Autoimmune Hemolytic Anemia (WAIHA) Phase 2** Data Trial Initiation 2022 2026 Planning for regular cadence of data across indications
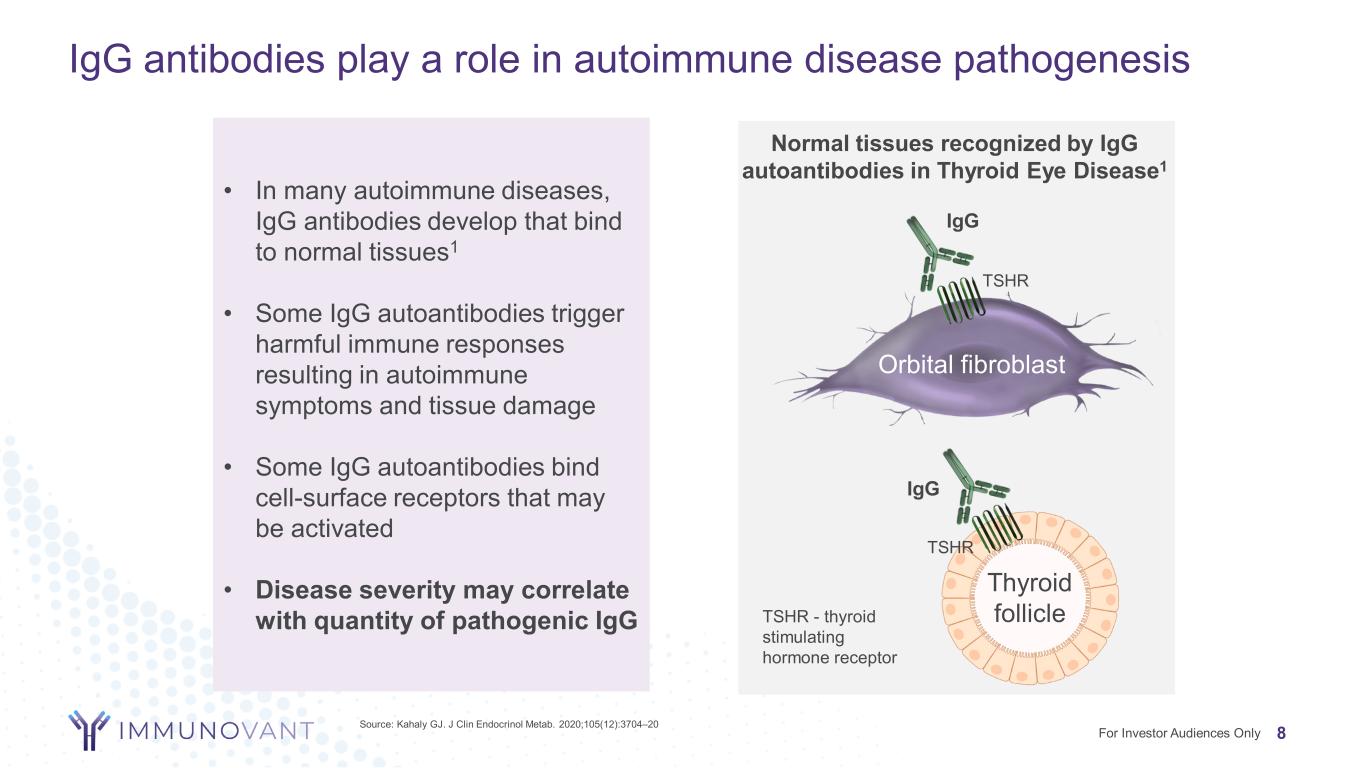
• In many autoimmune diseases, IgG antibodies develop that bind to normal tissues1 • Some IgG autoantibodies trigger harmful immune responses resulting in autoimmune symptoms and tissue damage • Some IgG autoantibodies bind cell-surface receptors that may be activated • Disease severity may correlate with quantity of pathogenic IgG IgG antibodies play a role in autoimmune disease pathogenesis Thyroid follicle Orbital fibroblast TSHR IgG Normal tissues recognized by IgG autoantibodies in Thyroid Eye Disease1 TSHR IgG Source: Kahaly GJ. J Clin Endocrinol Metab. 2020;105(12):3704–20 TSHR - thyroid stimulating hormone receptor 8For Investor Audiences Only
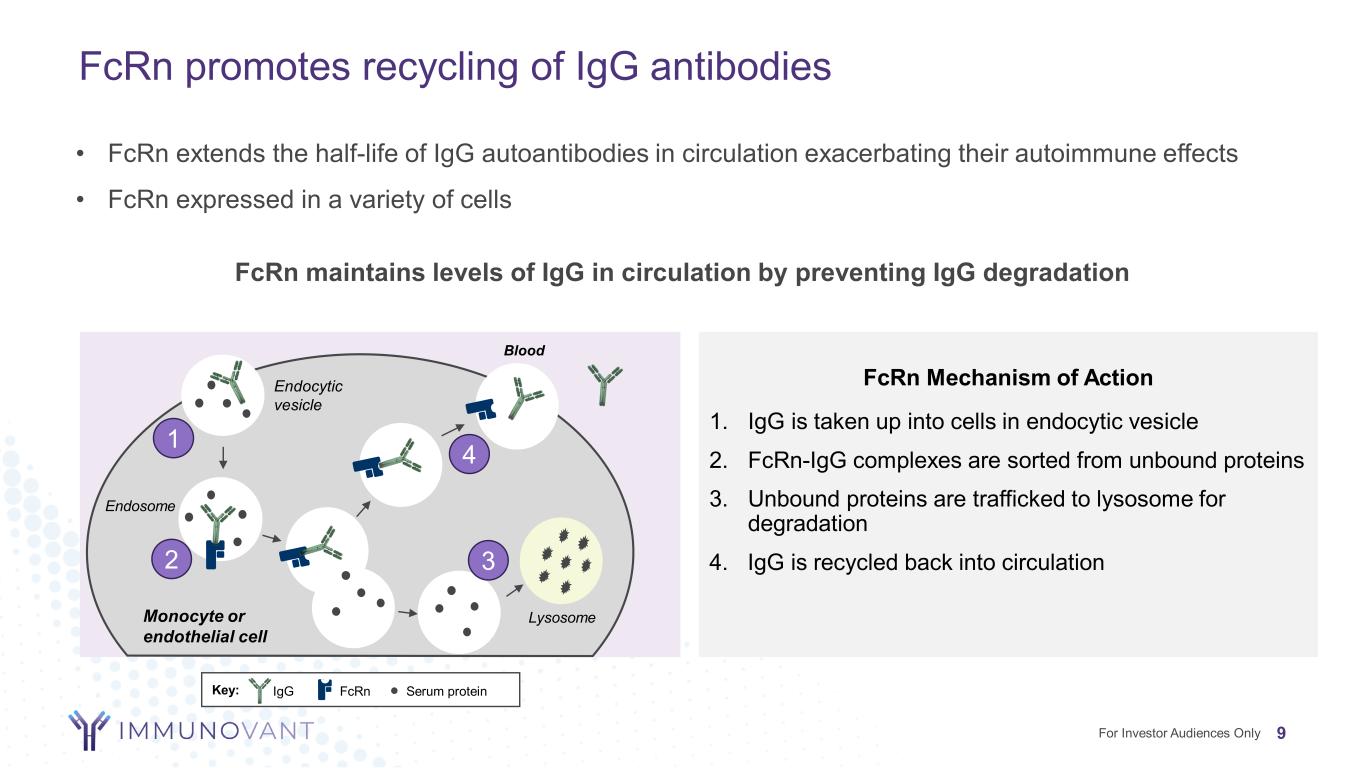
FcRn promotes recycling of IgG antibodies 9 FcRn Mechanism of Action 1. IgG is taken up into cells in endocytic vesicle 2. FcRn-IgG complexes are sorted from unbound proteins 3. Unbound proteins are trafficked to lysosome for degradation 4. IgG is recycled back into circulation FcRn maintains levels of IgG in circulation by preventing IgG degradation • FcRn extends the half-life of IgG autoantibodies in circulation exacerbating their autoimmune effects • FcRn expressed in a variety of cells Blood Endocytic vesicle Endosome LysosomeMonocyte or endothelial cell Key: Serum proteinIgG FcRn 1 2 3 4 For Investor Audiences Only
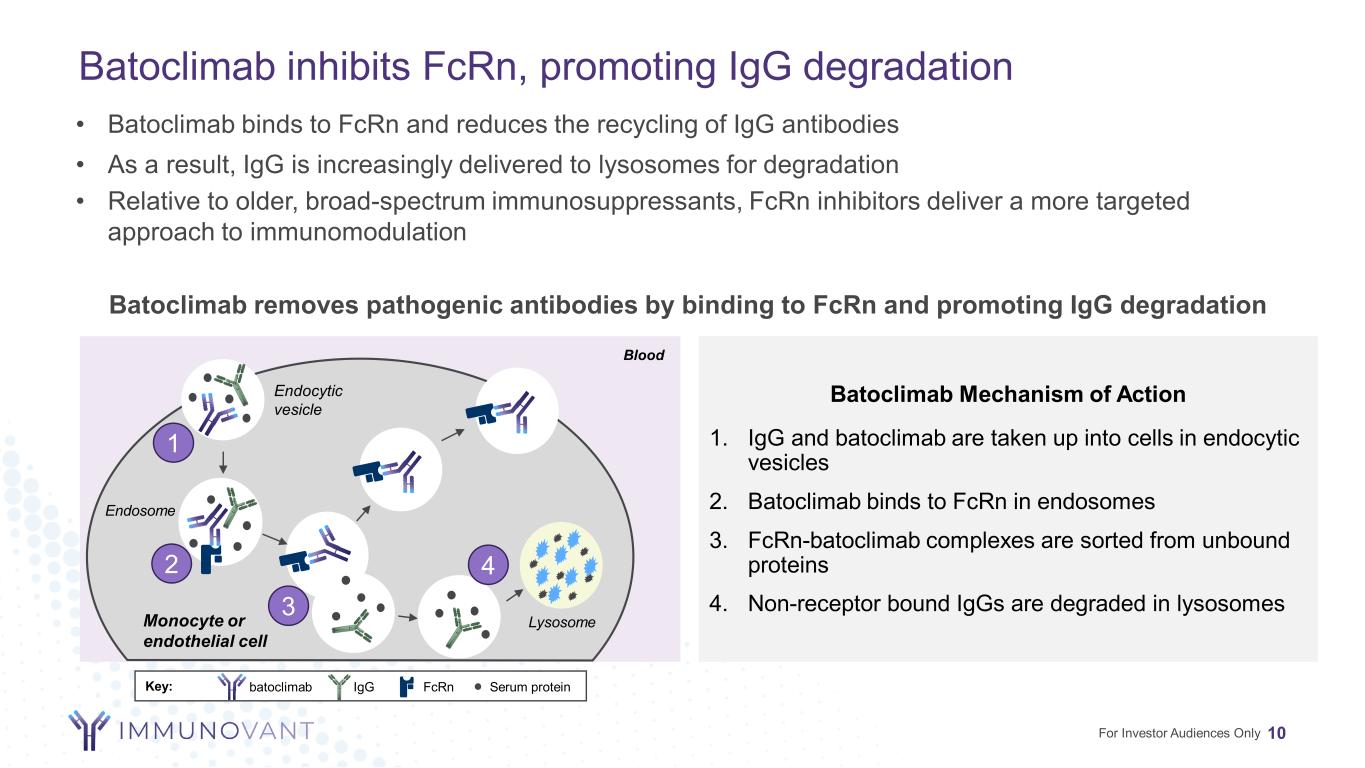
Blood Endocytic vesicle Endosome LysosomeMonocyte or endothelial cell Key: Serum proteinIgG FcRn 1 2 3 4 Batoclimab inhibits FcRn, promoting IgG degradation 10 Batoclimab Mechanism of Action 1. IgG and batoclimab are taken up into cells in endocytic vesicles 2. Batoclimab binds to FcRn in endosomes 3. FcRn-batoclimab complexes are sorted from unbound proteins 4. Non-receptor bound IgGs are degraded in lysosomes Batoclimab removes pathogenic antibodies by binding to FcRn and promoting IgG degradation • Batoclimab binds to FcRn and reduces the recycling of IgG antibodies • As a result, IgG is increasingly delivered to lysosomes for degradation • Relative to older, broad-spectrum immunosuppressants, FcRn inhibitors deliver a more targeted approach to immunomodulation batoclimab For Investor Audiences Only

1 1 Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) New Indication Announcement 2022
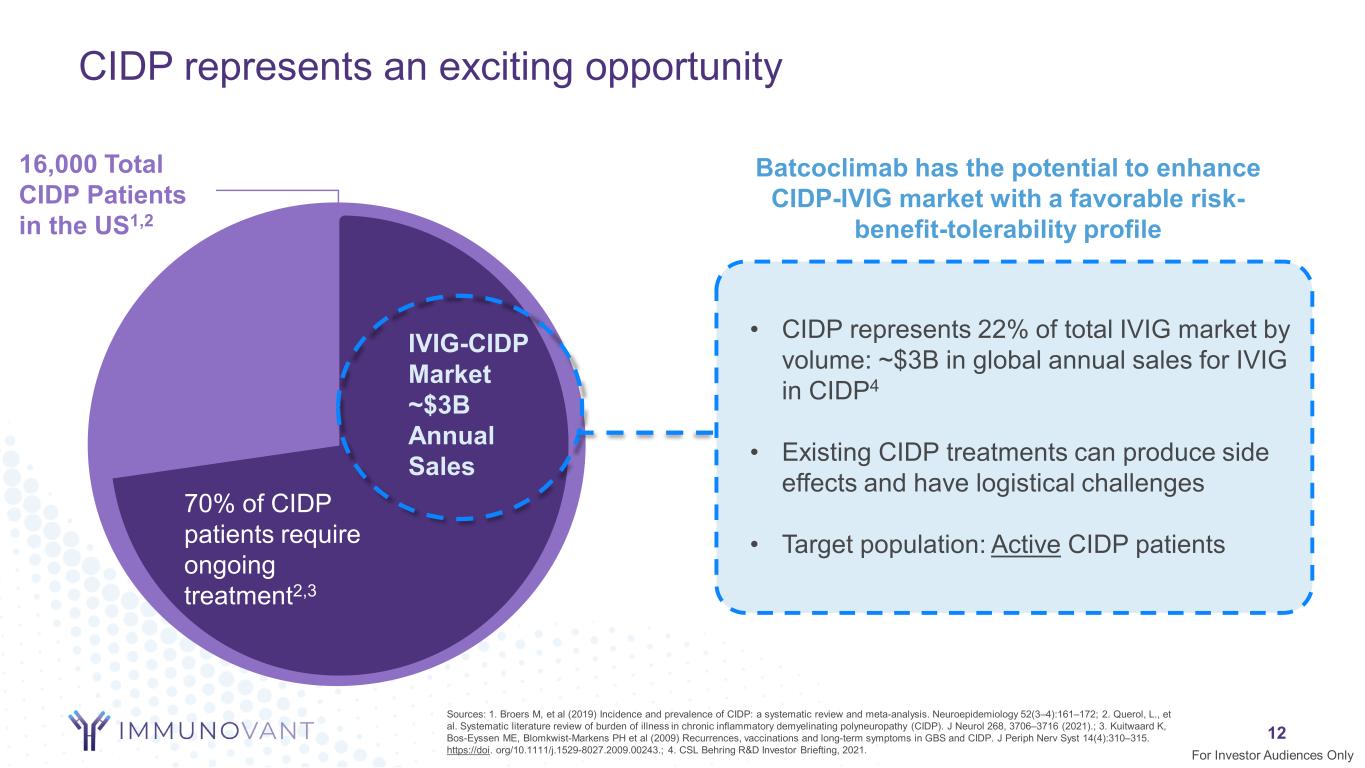
CIDP represents an exciting opportunity 12 Batcoclimab has the potential to enhance CIDP-IVIG market with a favorable risk- benefit-tolerability profile Sources: 1. Broers M, et al (2019) Incidence and prevalence of CIDP: a systematic review and meta-analysis. Neuroepidemiology 52(3–4):161–172; 2. Querol, L., et al. Systematic literature review of burden of illness in chronic inflammatory demyelinating polyneuropathy (CIDP). J Neurol 268, 3706–3716 (2021).; 3. Kuitwaard K, Bos-Eyssen ME, Blomkwist-Markens PH et al (2009) Recurrences, vaccinations and long-term symptoms in GBS and CIDP. J Periph Nerv Syst 14(4):310–315. https://doi. org/10.1111/j.1529-8027.2009.00243.; 4. CSL Behring R&D Investor Briefting, 2021. IVIG-CIDP Market ~$3B Annual Sales 16,000 Total CIDP Patients in the US1,2 • CIDP represents 22% of total IVIG market by volume: ~$3B in global annual sales for IVIG in CIDP4 • Existing CIDP treatments can produce side effects and have logistical challenges • Target population: Active CIDP patients 70% of CIDP patients require ongoing treatment2,3 For Investor Audiences Only

1 3 Fireside chat on CIDP Pete Salzmann, MD Chief Executive Officer, Immunovant Todd Levine, MD Medical Director, Neurology Department, Honor Health Scottsdale, Arizona Jonathan Katz, MD Director Neuromuscular Clinic, California Pacific Medical Center

Key Takeaways from CIDP Discussion 14 01 02 03 Diagnosis of CIDP is challenging and must be done carefully. Algorithms may improve diagnostic accuracy, which is paramount for clinical trial success. IVIG = intravenous immunoglobulin We believe CIDP is a very important disease in neurology and an exciting potential indication for the anti-FcRn class as current therapies (IVIG, PLEX and steroids) are effective, but have significant side effects and logistical limitations (IVIG & PLEX). Clinical trial design is also critical to maximize ability to demonstrate effect size. Patients entering CIDP trials after IVIG withdrawal are essentially untreated, whereas patients entering CIDP trials after steroid reduction remain partially treated and this may blunt the effect size observed in trials that include these patients within the primary analysis group. 14 04 For Investor Audiences Only CIDP is a chronic, symptomatic condition for many patients and therefore an effective treatment that could be administered via a simple subcutaneous injection would represent a meaningful improvement for patients with CIDP. We believe CIDP is ripe for disruption with the right mechanism, the right asset and the right trial design.
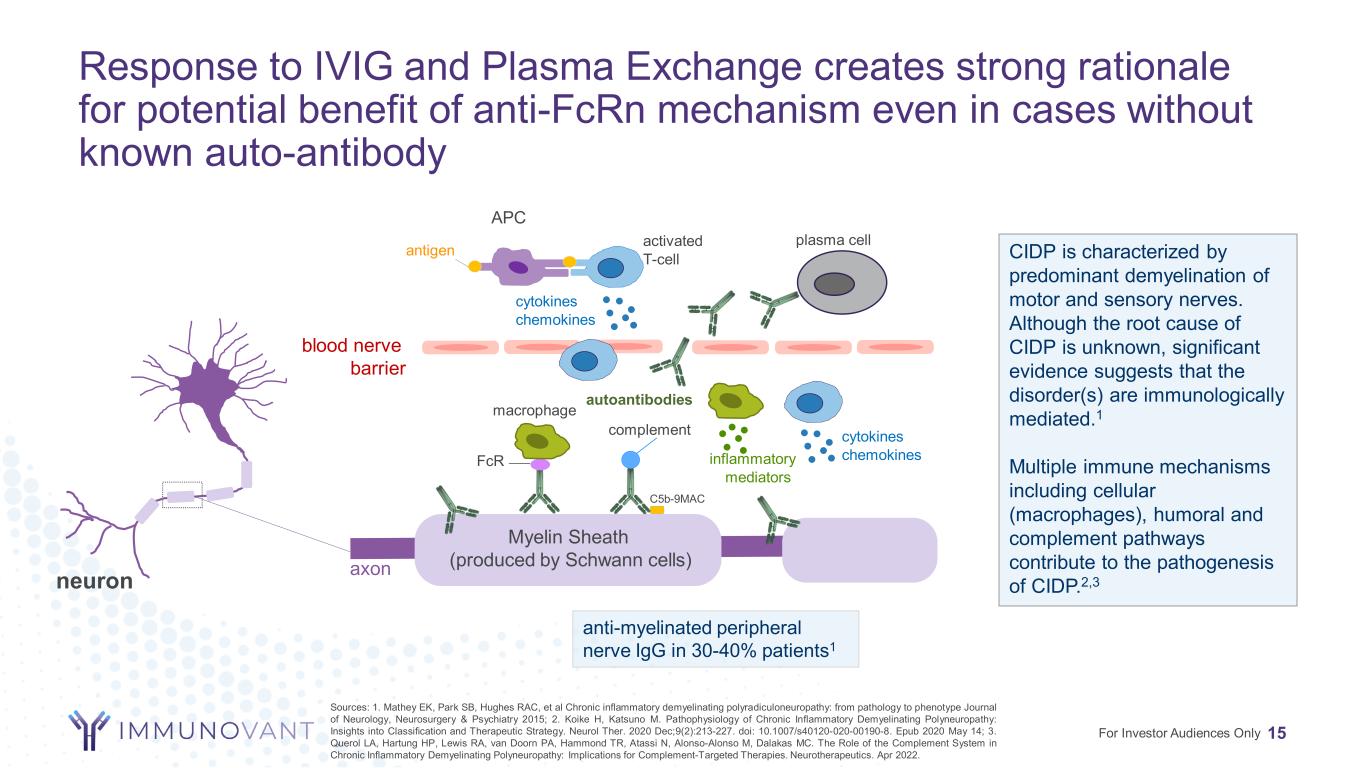
Response to IVIG and Plasma Exchange creates strong rationale for potential benefit of anti-FcRn mechanism even in cases without known auto-antibody 15 axon Myelin Sheath (produced by Schwann cells) anti-myelinated peripheral nerve IgG in 30-40% patients1 autoantibodies neuron CIDP is characterized by predominant demyelination of motor and sensory nerves. Although the root cause of CIDP is unknown, significant evidence suggests that the disorder(s) are immunologically mediated.1 Multiple immune mechanisms including cellular (macrophages), humoral and complement pathways contribute to the pathogenesis of CIDP.2,3 C5b-9MAC complement FcR plasma cell macrophage blood nerve barrier activated T-cell cytokines chemokinesinflammatory mediators APC antigen cytokines chemokines ’ For Investor Audiences Only Sources: 1. Mathey EK, Park SB, Hughes RAC, et al Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype Journal of Neurology, Neurosurgery & Psychiatry 2015; 2. Koike H, Katsuno M. Pathophysiology of Chronic Inflammatory Demyelinating Polyneuropathy: Insights into Classification and Therapeutic Strategy. Neurol Ther. 2020 Dec;9(2):213-227. doi: 10.1007/s40120-020-00190-8. Epub 2020 May 14; 3. Querol LA, Hartung HP, Lewis RA, van Doorn PA, Hammond TR, Atassi N, Alonso-Alonso M, Dalakas MC. The Role of the Complement System in Chronic Inflammatory Demyelinating Polyneuropathy: Implications for Complement-Targeted Therapies. Neurotherapeutics. Apr 2022.
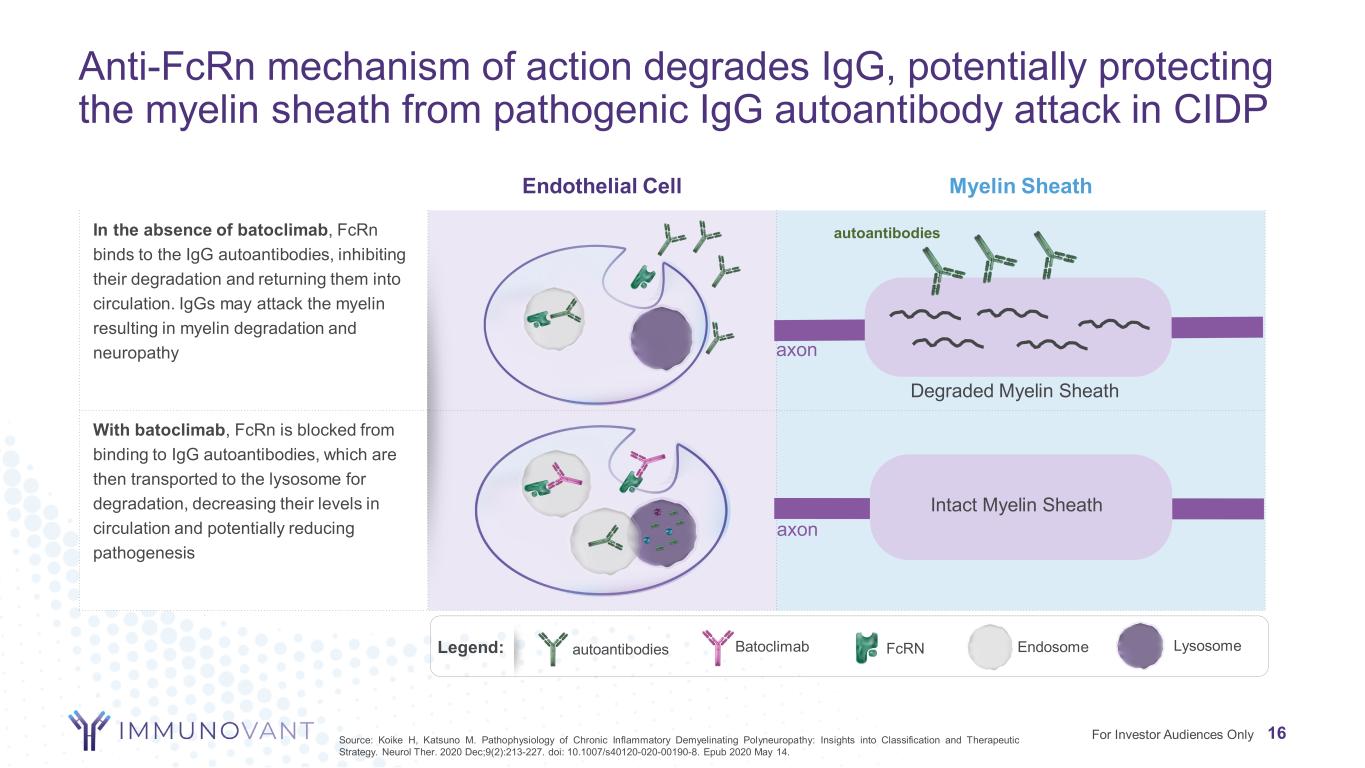
Anti-FcRn mechanism of action degrades IgG, potentially protecting the myelin sheath from pathogenic IgG autoantibody attack in CIDP 16 Endothelial Cell Myelin Sheath In the absence of batoclimab, FcRn binds to the IgG autoantibodies, inhibiting their degradation and returning them into circulation. IgGs may attack the myelin resulting in myelin degradation and neuropathy With batoclimab, FcRn is blocked from binding to IgG autoantibodies, which are then transported to the lysosome for degradation, decreasing their levels in circulation and potentially reducing pathogenesis autoantibodies Batoclimab FcRNLegend: axon Degraded Myelin Sheath axon Intact Myelin Sheath Lysosome autoantibodies Endosome For Investor Audiences OnlySource: Koike H, Katsuno M. Pathophysiology of Chronic Inflammatory Demyelinating Polyneuropathy: Insights into Classification and Therapeutic Strategy. Neurol Ther. 2020 Dec;9(2):213-227. doi: 10.1007/s40120-020-00190-8. Epub 2020 May 14.
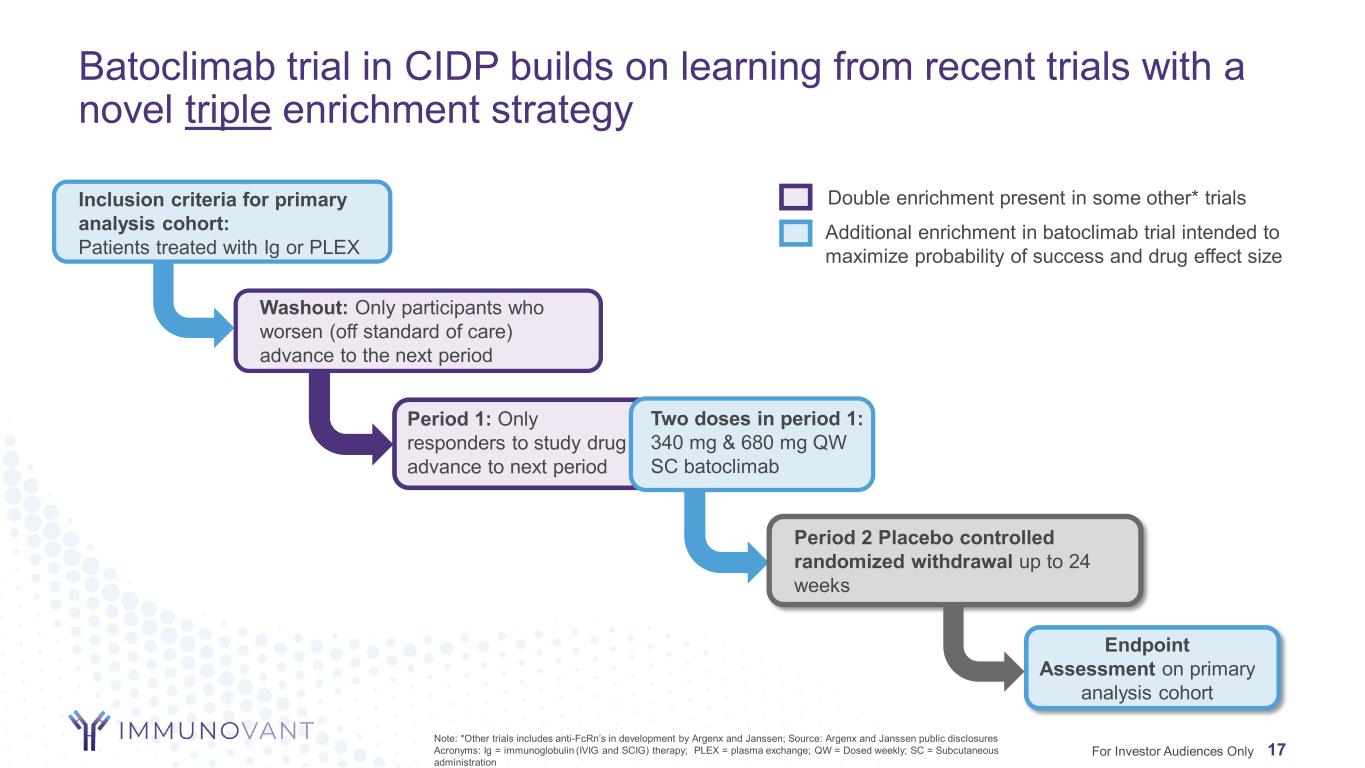
Batoclimab trial in CIDP builds on learning from recent trials with a novel triple enrichment strategy Inclusion criteria for primary analysis cohort: Patients treated with Ig or PLEX Washout: Only participants who worsen (off standard of care) advance to the next period Period 2 Placebo controlled randomized withdrawal up to 24 weeks Endpoint Assessment on primary analysis cohort Double enrichment present in some other* trials Additional enrichment in batoclimab trial intended to maximize probability of success and drug effect size Two doses in period 1: 340 mg & 680 mg QW SC batoclimab Period 1: Only responders to study drug advance to next period 17For Investor Audiences Only Note: *Other trials includes anti-FcRn’s in development by Argenx and Janssen; Source: Argenx and Janssen public disclosures Acronyms: Ig = immunoglobulin (IVIG and SCIG) therapy; PLEX = plasma exchange; QW = Dosed weekly; SC = Subcutaneous administration
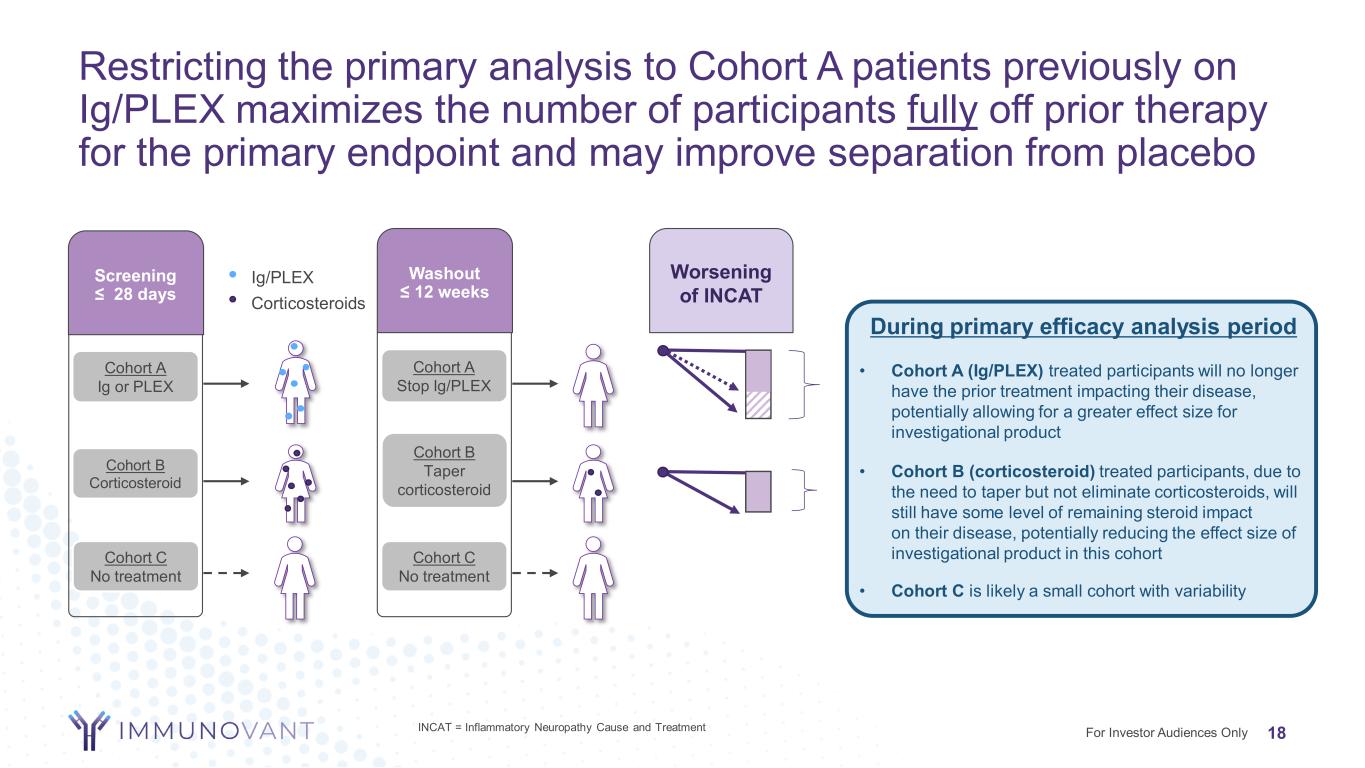
Restricting the primary analysis to Cohort A patients previously on Ig/PLEX maximizes the number of participants fully off prior therapy for the primary endpoint and may improve separation from placebo 18For Investor Audiences Only 3 cohorts Screening ≤ 28 days Washout ≤ 12 weeks Cohort A Ig or PLEX Cohort B Corticosteroid Cohort C No treatment Cohort A Stop Ig/PLEX Cohort B Taper corticosteroid Ig/PLEX Corticosteroids Cohort C No treatment During primary efficacy analysis period • Cohort A (Ig/PLEX) treated participants will no longer have the prior treatment impacting their disease, potentially allowing for a greater effect size for investigational product • Cohort B (corticosteroid) treated participants, due to the need to taper but not eliminate corticosteroids, will still have some level of remaining steroid impact on their disease, potentially reducing the effect size of investigational product in this cohort • Cohort C is likely a small cohort with variability Worsening of INCAT INCAT = Inflammatory Neuropathy Cause and Treatment
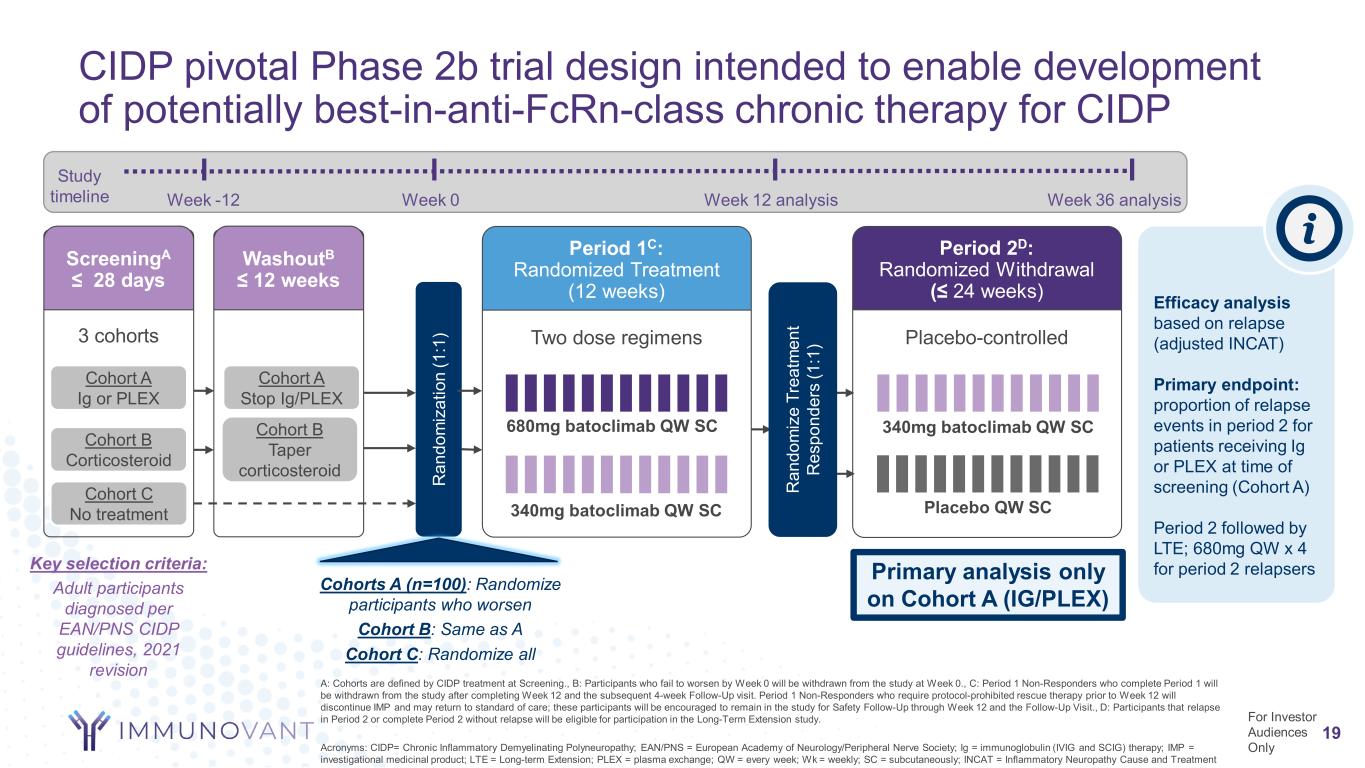
CIDP pivotal Phase 2b trial design intended to enable development of potentially best-in-anti-FcRn-class chronic therapy for CIDP 19 A: Cohorts are defined by CIDP treatment at Screening., B: Participants who fail to worsen by Week 0 will be withdrawn from the study at Week 0., C: Period 1 Non-Responders who complete Period 1 will be withdrawn from the study after completing Week 12 and the subsequent 4-week Follow-Up visit. Period 1 Non-Responders who require protocol-prohibited rescue therapy prior to Week 12 will discontinue IMP and may return to standard of care; these participants will be encouraged to remain in the study for Safety Follow-Up through Week 12 and the Follow-Up Visit., D: Participants that relapse in Period 2 or complete Period 2 without relapse will be eligible for participation in the Long-Term Extension study. Acronyms: CIDP= Chronic Inflammatory Demyelinating Polyneuropathy; EAN/PNS = European Academy of Neurology/Peripheral Nerve Society; Ig = immunoglobulin (IVIG and SCIG) therapy; IMP = investigational medicinal product; LTE = Long-term Extension; PLEX = plasma exchange; QW = every week; Wk = weekly; SC = subcutaneously; INCAT = Inflammatory Neuropathy Cause and Treatment Two dose regimens Period 1C: Randomized Treatment (12 weeks) Cohorts A (n=100): Randomize participants who worsen Cohort B: Same as A Cohort C: Randomize all Placebo-controlled Period 2D: Randomized Withdrawal (≤ 24 weeks) Placebo QW SC340mg batoclimab QW SC 680mg batoclimab QW SC 340mg batoclimab QW SC Efficacy analysis based on relapse (adjusted INCAT) Primary endpoint: proportion of relapse events in period 2 for patients receiving Ig or PLEX at time of screening (Cohort A) Period 2 followed by LTE; 680mg QW x 4 for period 2 relapsers R an do m iz e Tr ea tm en t R es po nd er s (1 :1 )3 cohorts ScreeningA ≤ 28 days WashoutB ≤ 12 weeks Cohort A Ig or PLEX Cohort B Corticosteroid Cohort C No treatment R an do m iz at io n (1 :1 ) Cohort A Stop Ig/PLEX Cohort B Taper corticosteroid Key selection criteria: Adult participants diagnosed per EAN/PNS CIDP guidelines, 2021 revision Week -12 Week 0 Week 12 analysis Week 36 analysis Study timeline For Investor Audiences Only Primary analysis only on Cohort A (IG/PLEX)
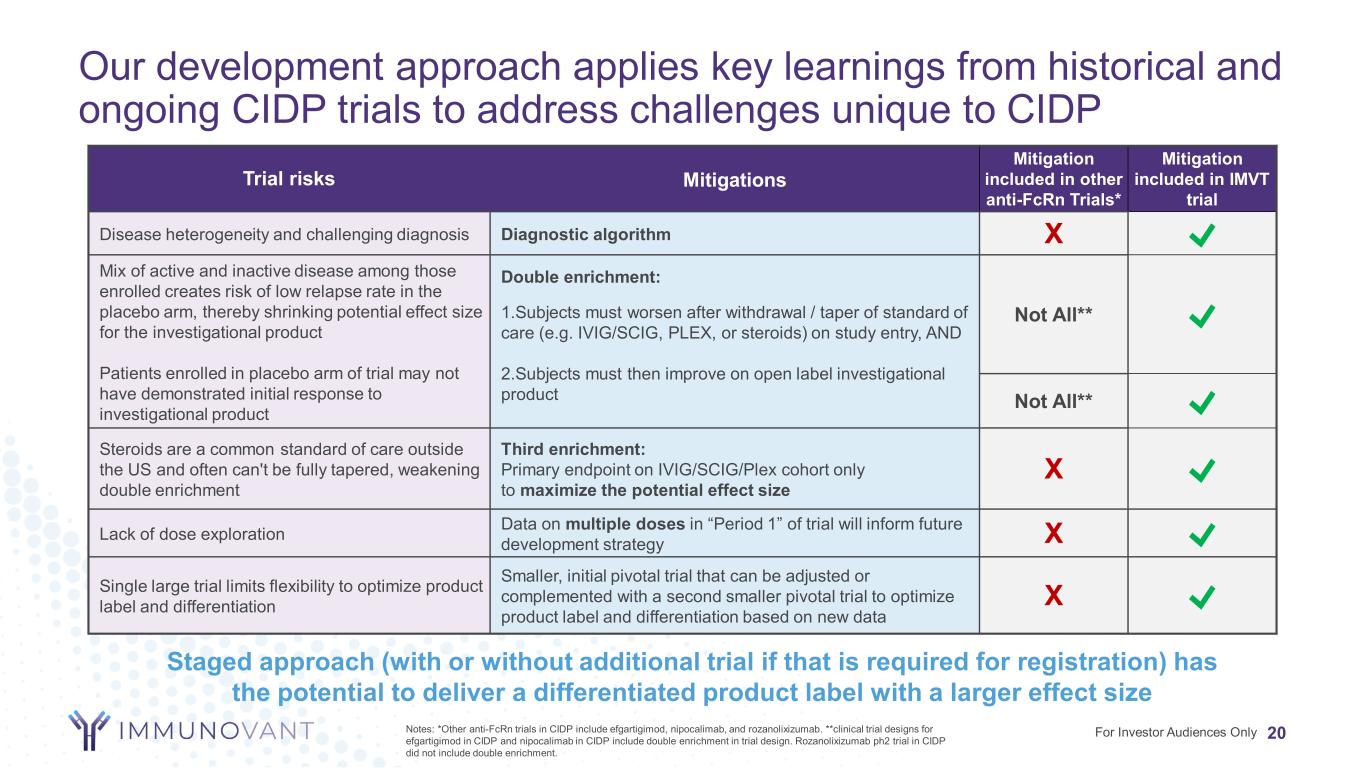
Our development approach applies key learnings from historical and ongoing CIDP trials to address challenges unique to CIDP 20For Investor Audiences Only Trial risks Mitigations Mitigation included in other anti-FcRn Trials* Mitigation included in IMVT trial Disease heterogeneity and challenging diagnosis Diagnostic algorithm X Mix of active and inactive disease among those enrolled creates risk of low relapse rate in the placebo arm, thereby shrinking potential effect size for the investigational product Patients enrolled in placebo arm of trial may not have demonstrated initial response to investigational product Double enrichment: Not All** 1.Subjects must worsen after withdrawal / taper of standard of care (e.g. IVIG/SCIG, PLEX, or steroids) on study entry, AND 2.Subjects must then improve on open label investigational product Not All** Steroids are a common standard of care outside the US and often can't be fully tapered, weakening double enrichment Third enrichment: Primary endpoint on IVIG/SCIG/Plex cohort only to maximize the potential effect size X Lack of dose exploration Data on multiple doses in “Period 1” of trial will inform future development strategy X Single large trial limits flexibility to optimize product label and differentiation Smaller, initial pivotal trial that can be adjusted or complemented with a second smaller pivotal trial to optimize product label and differentiation based on new data X Notes: *Other anti-FcRn trials in CIDP include efgartigimod, nipocalimab, and rozanolixizumab. **clinical trial designs for efgartigimod in CIDP and nipocalimab in CIDP include double enrichment in trial design. Rozanolixizumab ph2 trial in CIDP did not include double enrichment. Staged approach (with or without additional trial if that is required for registration) has the potential to deliver a differentiated product label with a larger effect size
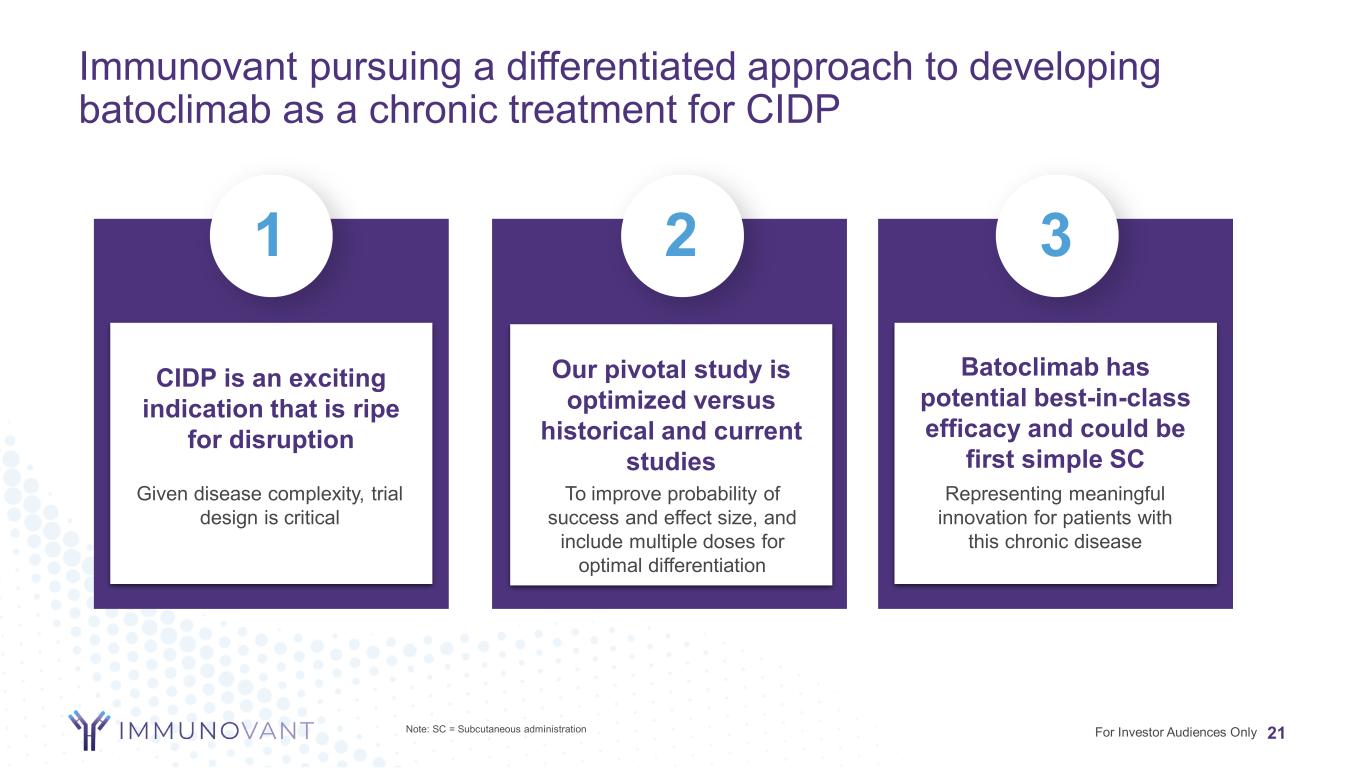
Immunovant pursuing a differentiated approach to developing batoclimab as a chronic treatment for CIDP 21 CIDP is an exciting indication that is ripe for disruption Our pivotal study is optimized versus historical and current studies Batoclimab has potential best-in-class efficacy and could be first simple SC 1 2 3 For Investor Audiences Only Representing meaningful innovation for patients with this chronic disease To improve probability of success and effect size, and include multiple doses for optimal differentiation Given disease complexity, trial design is critical Note: SC = Subcutaneous administration

2 2 Graves' Disease New Indication Announcement 2022
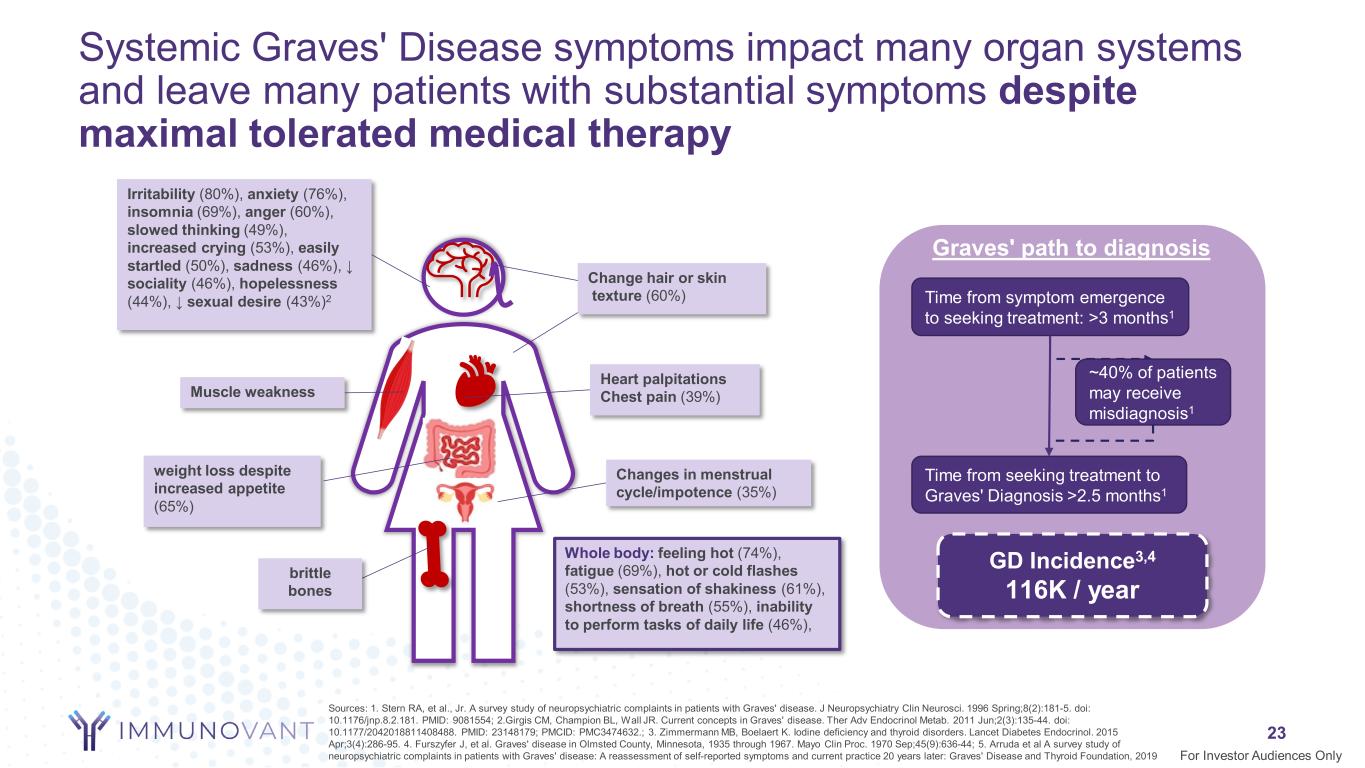
Systemic Graves' Disease symptoms impact many organ systems and leave many patients with substantial symptoms despite maximal tolerated medical therapy 23 Sources: 1. Stern RA, et al., Jr. A survey study of neuropsychiatric complaints in patients with Graves' disease. J Neuropsychiatry Clin Neurosci. 1996 Spring;8(2):181-5. doi: 10.1176/jnp.8.2.181. PMID: 9081554; 2.Girgis CM, Champion BL, Wall JR. Current concepts in Graves' disease. Ther Adv Endocrinol Metab. 2011 Jun;2(3):135-44. doi: 10.1177/2042018811408488. PMID: 23148179; PMCID: PMC3474632.; 3. Zimmermann MB, Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015 Apr;3(4):286-95. 4. Furszyfer J, et al. Graves' disease in Olmsted County, Minnesota, 1935 through 1967. Mayo Clin Proc. 1970 Sep;45(9):636-44; 5. Arruda et al A survey study of neuropsychiatric complaints in patients with Graves' disease: A reassessment of self-reported symptoms and current practice 20 years later: Graves' Disease and Thyroid Foundation, 2019 Time from symptom emergence to seeking treatment: >3 months1 Time from seeking treatment to Graves' Diagnosis >2.5 months1 GD Incidence3,4 116K / year For Investor Audiences Only Irritability (80%), anxiety (76%), insomnia (69%), anger (60%), slowed thinking (49%), increased crying (53%), easily startled (50%), sadness (46%), ↓ sociality (46%), hopelessness (44%), ↓ sexual desire (43%)2 weight loss despite increased appetite (65%) Whole body: feeling hot (74%), fatigue (69%), hot or cold flashes (53%), sensation of shakiness (61%), shortness of breath (55%), inability to perform tasks of daily life (46%), brittle bones Muscle weakness Change hair or skin texture (60%) Heart palpitations Chest pain (39%) Changes in menstrual cycle/impotence (35%) ~40% of patients may receive misdiagnosis1 Graves' path to diagnosis
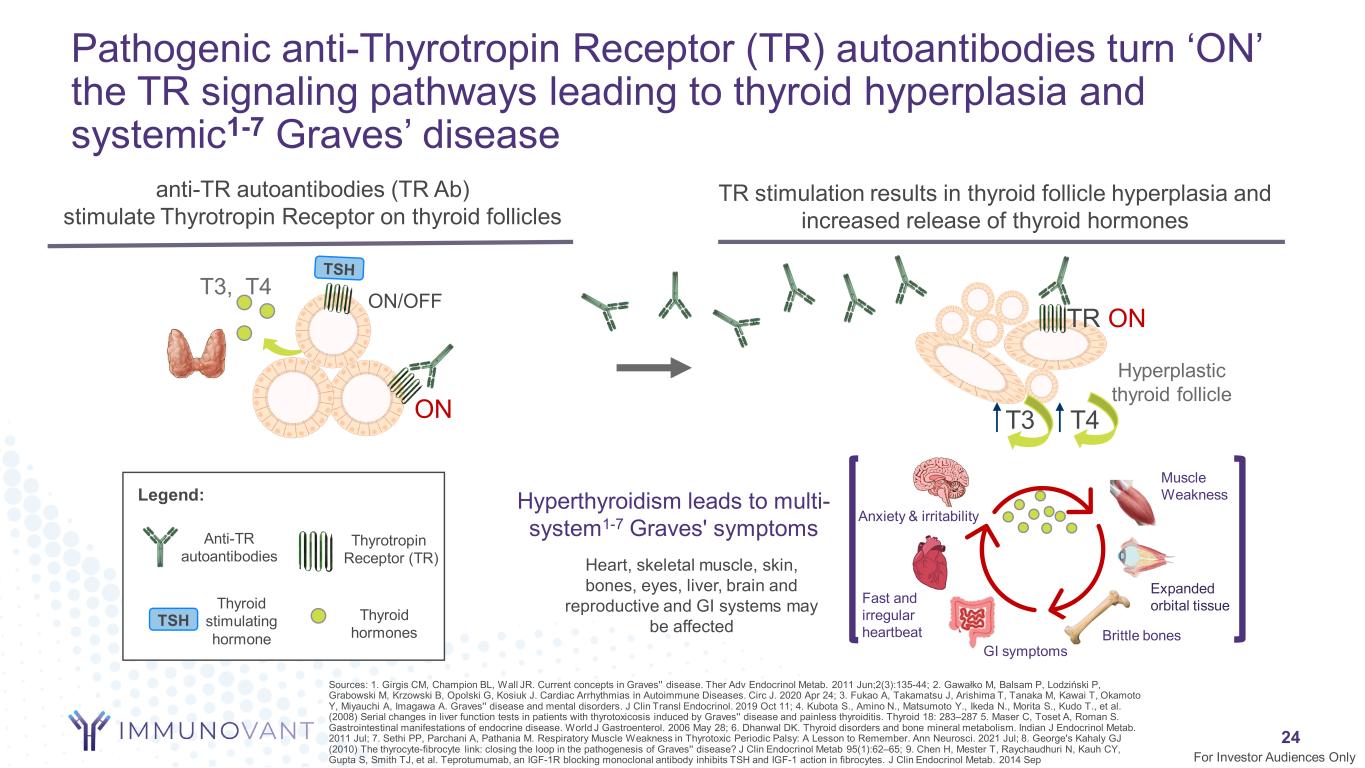
24 anti-TR autoantibodies (TR Ab) stimulate Thyrotropin Receptor on thyroid follicles Hyperplastic thyroid follicle T3 T4ON TR ON T3, T4 ON/OFF Pathogenic anti-Thyrotropin Receptor (TR) autoantibodies turn ‘ON’ the TR signaling pathways leading to thyroid hyperplasia and systemic1-7 Graves’ disease Anti-TR autoantibodies Thyroid stimulating hormone Legend: TSH Thyrotropin Receptor (TR) For Investor Audiences Only TR stimulation results in thyroid follicle hyperplasia and increased release of thyroid hormones Expanded orbital tissue Brittle bones GI symptoms Fast and irregular heartbeat Anxiety & irritability Muscle WeaknessHyperthyroidism leads to multi- system1-7 Graves' symptoms Heart, skeletal muscle, skin, bones, eyes, liver, brain and reproductive and GI systems may be affected Thyroid hormones Sources: 1. Girgis CM, Champion BL, Wall JR. Current concepts in Graves'' disease. Ther Adv Endocrinol Metab. 2011 Jun;2(3):135-44; 2. Gawałko M, Balsam P, Lodziński P, Grabowski M, Krzowski B, Opolski G, Kosiuk J. Cardiac Arrhythmias in Autoimmune Diseases. Circ J. 2020 Apr 24; 3. Fukao A, Takamatsu J, Arishima T, Tanaka M, Kawai T, Okamoto Y, Miyauchi A, Imagawa A. Graves'' disease and mental disorders. J Clin Transl Endocrinol. 2019 Oct 11; 4. Kubota S., Amino N., Matsumoto Y., Ikeda N., Morita S., Kudo T., et al. (2008) Serial changes in liver function tests in patients with thyrotoxicosis induced by Graves'' disease and painless thyroiditis. Thyroid 18: 283–287 5. Maser C, Toset A, Roman S. Gastrointestinal manifestations of endocrine disease. World J Gastroenterol. 2006 May 28; 6. Dhanwal DK. Thyroid disorders and bone mineral metabolism. Indian J Endocrinol Metab. 2011 Jul; 7. Sethi PP, Parchani A, Pathania M. Respiratory Muscle Weakness in Thyrotoxic Periodic Palsy: A Lesson to Remember. Ann Neurosci. 2021 Jul; 8. George's Kahaly GJ (2010) The thyrocyte-fibrocyte link: closing the loop in the pathogenesis of Graves'' disease? J Clin Endocrinol Metab 95(1):62–65; 9. Chen H, Mester T, Raychaudhuri N, Kauh CY, Gupta S, Smith TJ, et al. Teprotumumab, an IGF-1R blocking monoclonal antibody inhibits TSH and IGF-1 action in fibrocytes. J Clin Endocrinol Metab. 2014 Sep
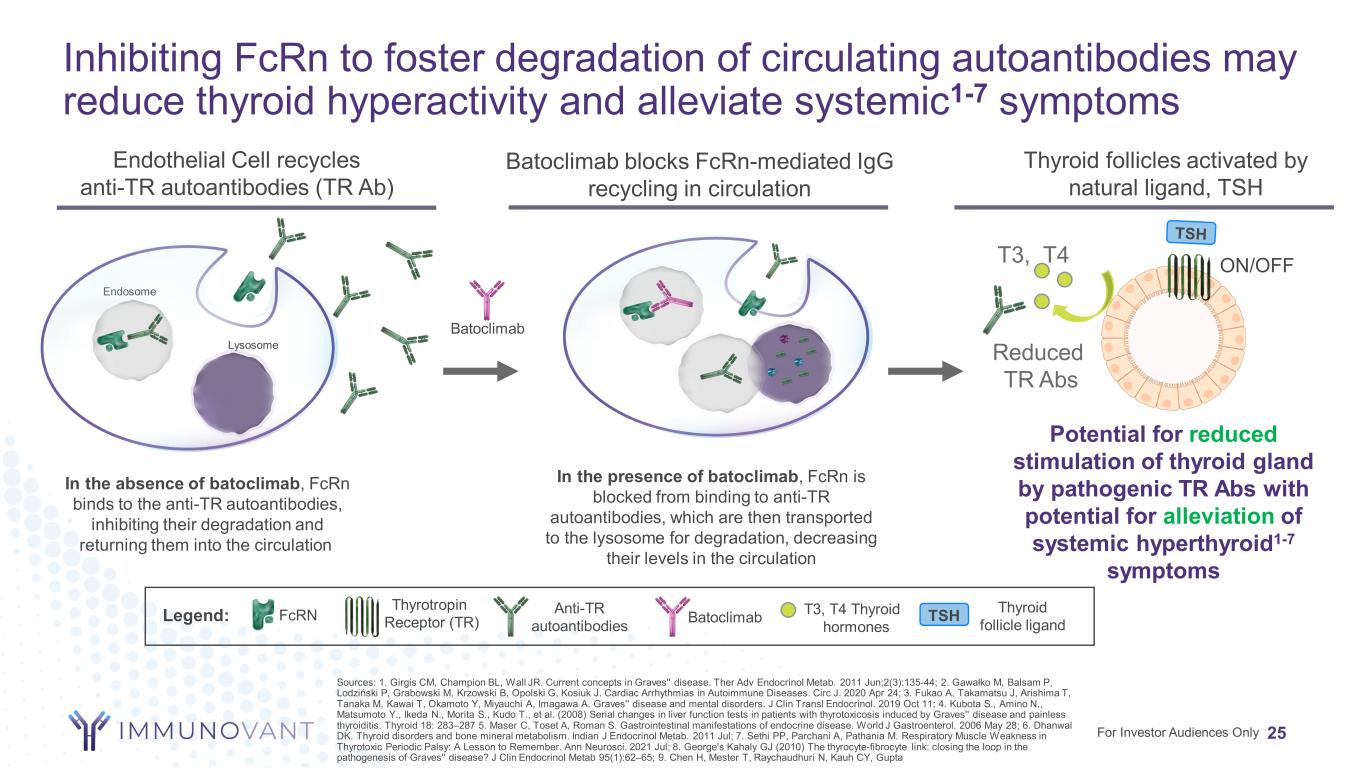
Inhibiting FcRn to foster degradation of circulating autoantibodies may reduce thyroid hyperactivity and alleviate systemic1-7 symptoms 25 Anti-TR autoantibodies FcRNLegend: Batoclimab blocks FcRn-mediated IgG recycling in circulation Thyroid follicles activated by natural ligand, TSH Reduced TR Abs T3, T4 In the presence of batoclimab, FcRn is blocked from binding to anti-TR autoantibodies, which are then transported to the lysosome for degradation, decreasing their levels in the circulation Batoclimab Potential for reduced stimulation of thyroid gland by pathogenic TR Abs with potential for alleviation of systemic hyperthyroid1-7 symptoms For Investor Audiences Only In the absence of batoclimab, FcRn binds to the anti-TR autoantibodies, inhibiting their degradation and returning them into the circulation Endosome Lysosome Batoclimab Thyrotropin Receptor (TR) ON/OFF Endothelial Cell recycles anti-TR autoantibodies (TR Ab) T3, T4 Thyroid hormones Sources: 1. Girgis CM, Champion BL, Wall JR. Current concepts in Graves'' disease. Ther Adv Endocrinol Metab. 2011 Jun;2(3):135-44; 2. Gawałko M, Balsam P, Lodziński P, Grabowski M, Krzowski B, Opolski G, Kosiuk J. Cardiac Arrhythmias in Autoimmune Diseases. Circ J. 2020 Apr 24; 3. Fukao A, Takamatsu J, Arishima T, Tanaka M, Kawai T, Okamoto Y, Miyauchi A, Imagawa A. Graves'' disease and mental disorders. J Clin Transl Endocrinol. 2019 Oct 11; 4. Kubota S., Amino N., Matsumoto Y., Ikeda N., Morita S., Kudo T., et al. (2008) Serial changes in liver function tests in patients with thyrotoxicosis induced by Graves'' disease and painless thyroiditis. Thyroid 18: 283–287 5. Maser C, Toset A, Roman S. Gastrointestinal manifestations of endocrine disease. World J Gastroenterol. 2006 May 28; 6. Dhanwal DK. Thyroid disorders and bone mineral metabolism. Indian J Endocrinol Metab. 2011 Jul; 7. Sethi PP, Parchani A, Pathania M. Respiratory Muscle Weakness in Thyrotoxic Periodic Palsy: A Lesson to Remember. Ann Neurosci. 2021 Jul; 8. George's Kahaly GJ (2010) The thyrocyte-fibrocyte link: closing the loop in the pathogenesis of Graves'' disease? J Clin Endocrinol Metab 95(1):62–65; 9. Chen H, Mester T, Raychaudhuri N, Kauh CY, Gupta TSH Thyroid follicle ligand
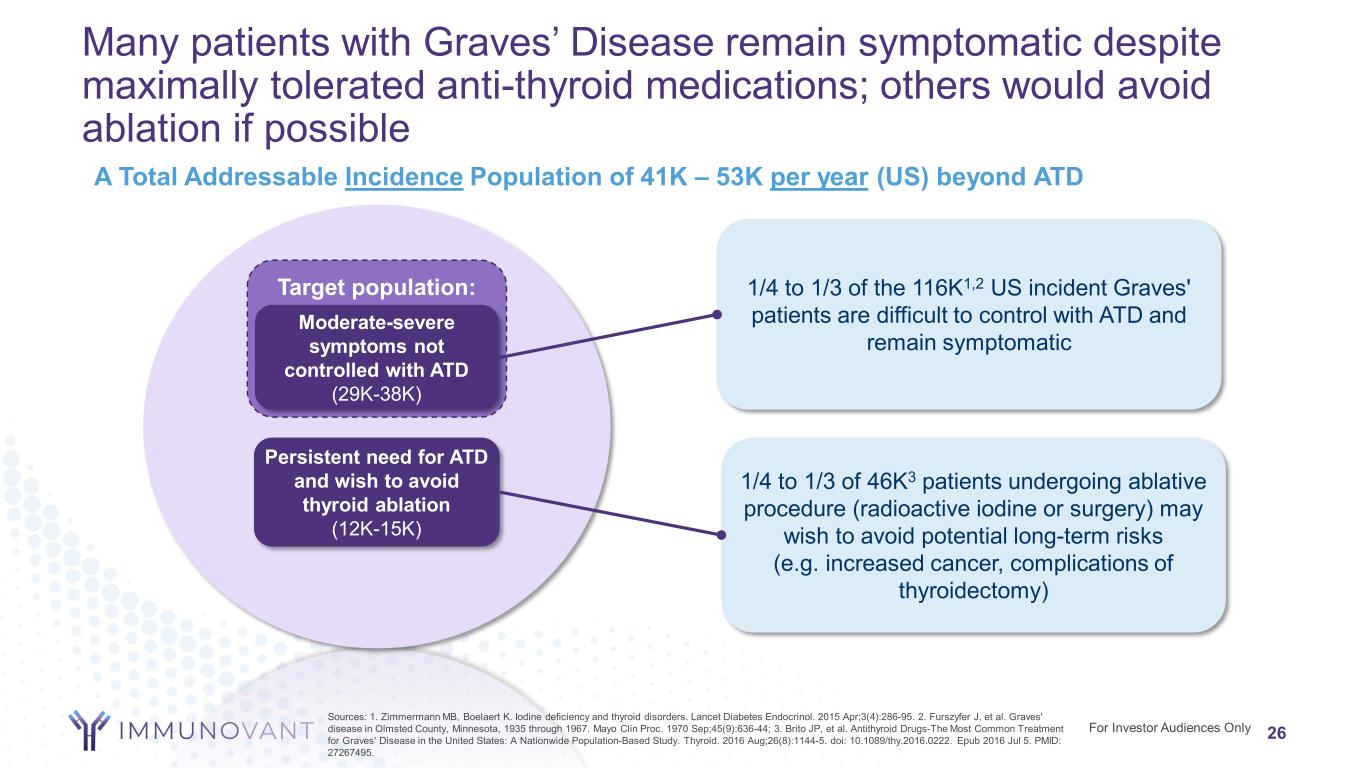
Target population: 26 A Total Addressable Incidence Population of 41K – 53K per year (US) beyond ATD Persistent need for ATD and wish to avoid thyroid ablation (12K-15K) Moderate-severe symptoms not controlled with ATD (29K-38K) Sources: 1. Zimmermann MB, Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015 Apr;3(4):286-95. 2. Furszyfer J, et al. Graves' disease in Olmsted County, Minnesota, 1935 through 1967. Mayo Clin Proc. 1970 Sep;45(9):636-44; 3. Brito JP, et al. Antithyroid Drugs-The Most Common Treatment for Graves' Disease in the United States: A Nationwide Population-Based Study. Thyroid. 2016 Aug;26(8):1144-5. doi: 10.1089/thy.2016.0222. Epub 2016 Jul 5. PMID: 27267495. For Investor Audiences Only 1/4 to 1/3 of the 116K1,2 US incident Graves' patients are difficult to control with ATD and remain symptomatic 1/4 to 1/3 of 46K3 patients undergoing ablative procedure (radioactive iodine or surgery) may wish to avoid potential long-term risks (e.g. increased cancer, complications of thyroidectomy) Many patients with Graves’ Disease remain symptomatic despite maximally tolerated anti-thyroid medications; others would avoid ablation if possible
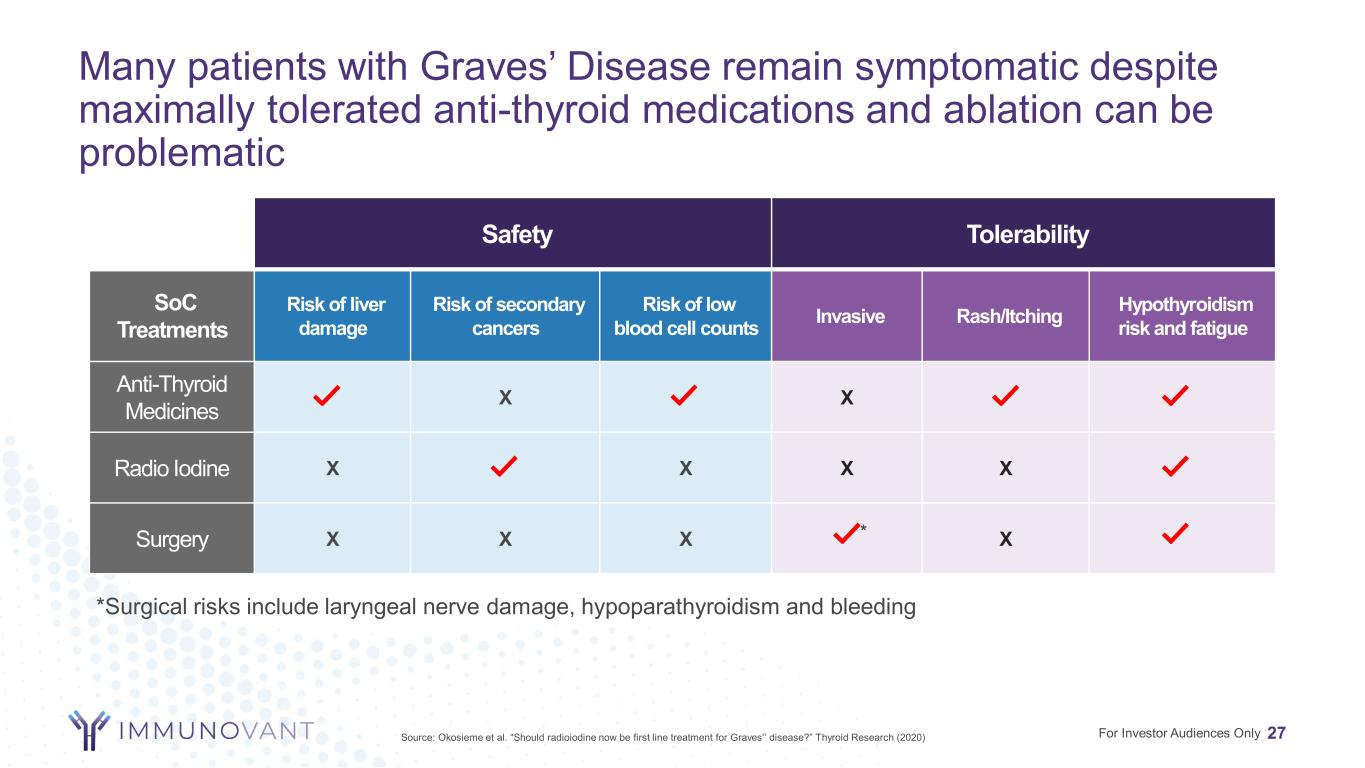
Many patients with Graves’ Disease remain symptomatic despite maximally tolerated anti-thyroid medications and ablation can be problematic 27 Safety Tolerability SoC Treatments Risk of liver damage Risk of secondary cancers Risk of low blood cell counts Invasive Rash/Itching Hypothyroidism risk and fatigue Anti-Thyroid Medicines X X Radio Iodine X X X X Surgery X X X * X For Investor Audiences OnlySource: Okosieme et al. “Should radioiodine now be first line treatment for Graves'’ disease?” Thyroid Research (2020) *Surgical risks include laryngeal nerve damage, hypoparathyroidism and bleeding
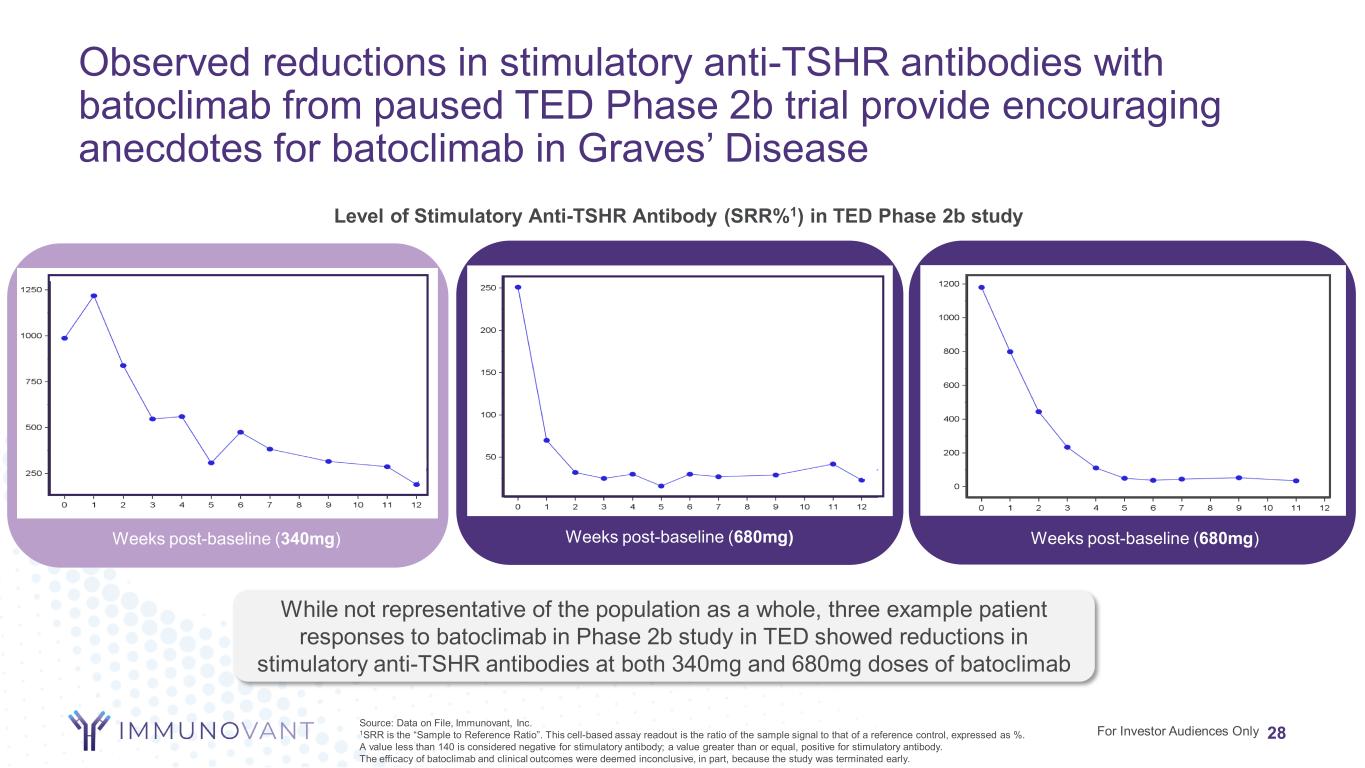
Observed reductions in stimulatory anti-TSHR antibodies with batoclimab from paused TED Phase 2b trial provide encouraging anecdotes for batoclimab in Graves’ Disease 28For Investor Audiences Only Source: Data on File, Immunovant, Inc. 1SRR is the “Sample to Reference Ratio”. This cell-based assay readout is the ratio of the sample signal to that of a reference control, expressed as %. A value less than 140 is considered negative for stimulatory antibody; a value greater than or equal, positive for stimulatory antibody. The efficacy of batoclimab and clinical outcomes were deemed inconclusive, in part, because the study was terminated early. Weeks post-baseline (680mg)Weeks post-baseline (340mg) Level of Stimulatory Anti-TSHR Antibody (SRR%1) in TED Phase 2b study Weeks post-baseline (680mg) While not representative of the population as a whole, three example patient responses to batoclimab in Phase 2b study in TED showed reductions in stimulatory anti-TSHR antibodies at both 340mg and 680mg doses of batoclimab

2 9 Fireside chat on Graves' Disease Pete Salzmann, MD Chief Executive Officer, Immunovant George J. Kahaly, MD, PhD Professor of Medicine and Endocrinology/Metabolism Johannes Gutenberg University (JGU) Medical Center Department of Medicine I ORPHAN Disease Center for Graves' Orbitopathy and Autoimmune Polyendocrinopathy

Key Takeaways from Graves' Disease Discussion 01 02 03 Diverse and bothersome symptoms primarily relate to elevated thyroid hormone levels (measured as increased T3 and T4) are a direct result of overstimulation of the thyroid gland by anti-TSHR auto-antibodies Standard of care for Graves' Disease is often limited by safety and tolerability concerns, leaving many patients needing additional efficacy to control their thyroid hormone levels and symptoms FcRn inhibition lowers total and pathogenic IgG and therefore has the potential to significantly improve signs and symptoms in patients who insufficiently respond to anti-thyroid drugs and want to avoid ablative therapy For Investor Audiences Only 30
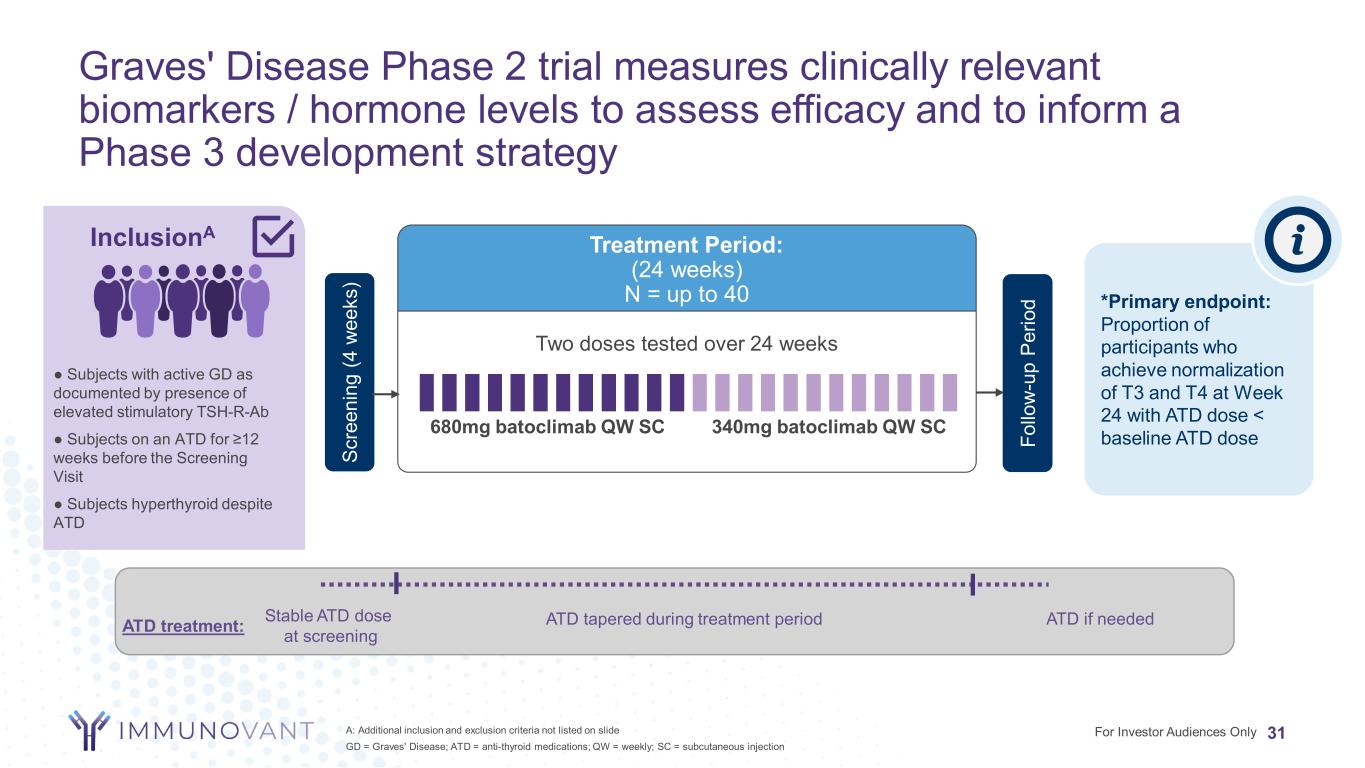
Graves' Disease Phase 2 trial measures clinically relevant biomarkers / hormone levels to assess efficacy and to inform a Phase 3 development strategy 31A: Additional inclusion and exclusion criteria not listed on slide GD = Graves' Disease; ATD = anti-thyroid medications; QW = weekly; SC = subcutaneous injection Two doses tested over 24 weeks Treatment Period: (24 weeks) N = up to 40 340mg batoclimab QW SC680mg batoclimab QW SC *Primary endpoint: Proportion of participants who achieve normalization of T3 and T4 at Week 24 with ATD dose < baseline ATD doseFo llo w -u p Pe rio d InclusionA ● Subjects with active GD as documented by presence of elevated stimulatory TSH-R-Ab ● Subjects on an ATD for ≥12 weeks before the Screening Visit ● Subjects hyperthyroid despite ATD Sc re en in g (4 w ee ks ) ATD treatment: Stable ATD dose at screening ATD tapered during treatment period ATD if needed For Investor Audiences Only
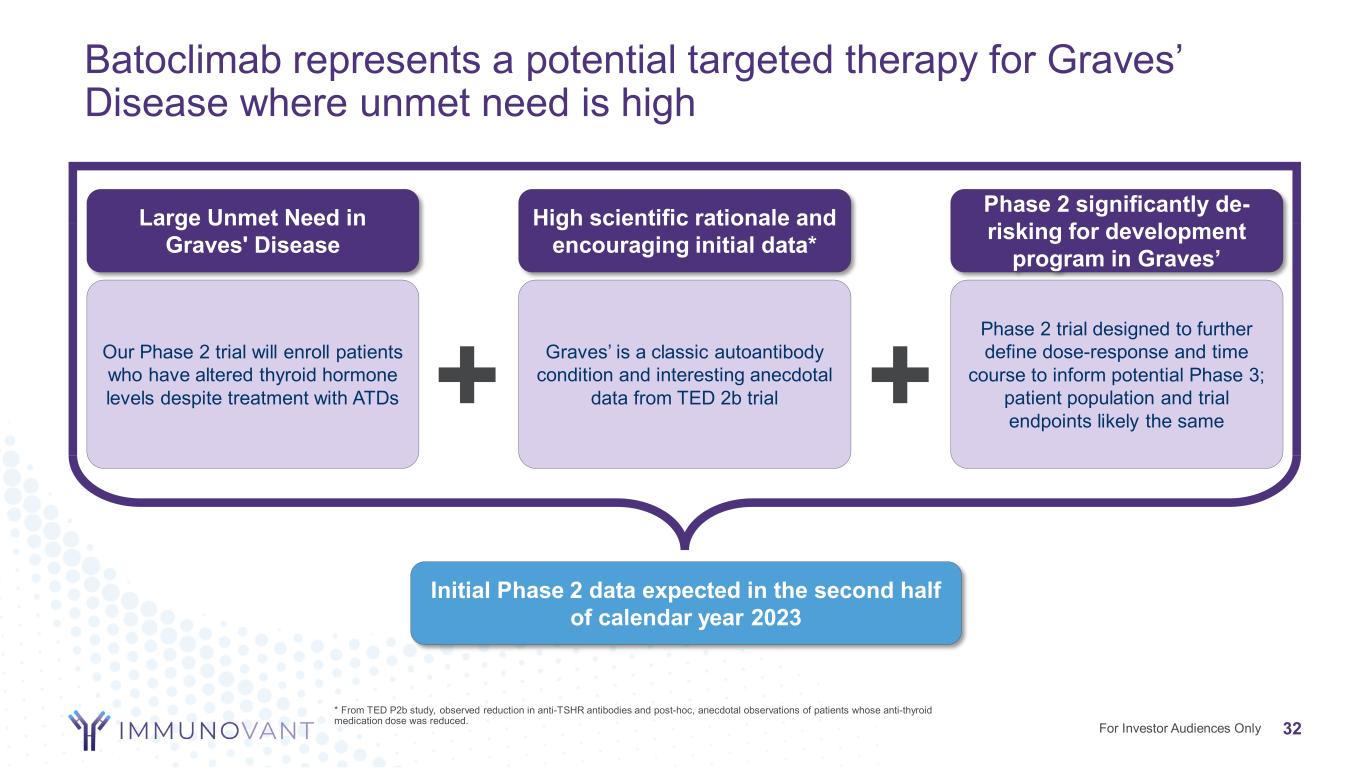
Batoclimab represents a potential targeted therapy for Graves’ Disease where unmet need is high For Investor Audiences Only * From TED P2b study, observed reduction in anti-TSHR antibodies and post-hoc, anecdotal observations of patients whose anti-thyroid medication dose was reduced. Initial Phase 2 data expected in the second half of calendar year 2023 Large Unmet Need in Graves' Disease Our Phase 2 trial will enroll patients who have altered thyroid hormone levels despite treatment with ATDs High scientific rationale and encouraging initial data* Graves’ is a classic autoantibody condition and interesting anecdotal data from TED 2b trial Phase 2 significantly de- risking for development program in Graves’ Phase 2 trial designed to further define dose-response and time course to inform potential Phase 3; patient population and trial endpoints likely the same+ + 32

Closing 33

Pipeline expansion: Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) & Graves' Disease 34 01 02 03 SC = Subcutaneous administration; MG = Myasthenia Gravis; TED = Thyroid Eye Disease 1. As of June 30, 2022, per most recent Quarterly Report on Form 10-Q filed with the SEC on August 5, 2022 2. The assumptions upon which we have based our estimates, including expenditures relating to planned or potential clinical trials, are routinely evaluated and may be subject to change 34For Investor Audiences Only Data catalysts for new indications complement pivotal data from MG and TED programs. Expect cadence of data every half year starting in the second half of 2023 Graves’ Disease is a first-in-class opportunity in an autoantibody driven condition with a straightforward Phase 2 approach and a high unmet need in a large subset of patients insufficiently responding even at maximally tolerated doses of current standard of care CIDP is an exciting opportunity for the anti-FcRn class. We believe that our uniquely optimized trial could position batoclimab with best-in-class efficacy and first-to-market simple SC 04 Immunovant has $427M in cash with cash runway into 20251,2
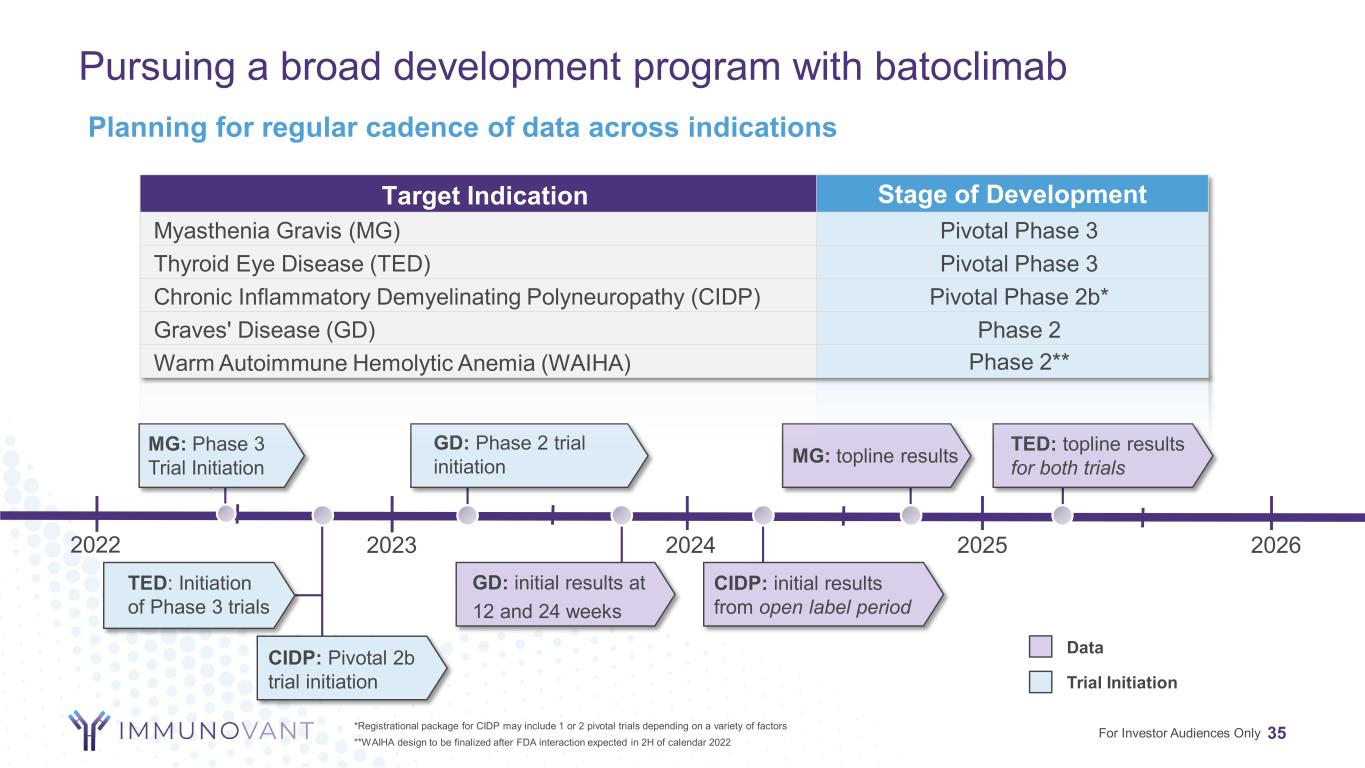
Pursuing a broad development program with batoclimab 35*Registrational package for CIDP may include 1 or 2 pivotal trials depending on a variety of factors **WAIHA design to be finalized after FDA interaction expected in 2H of calendar 2022 For Investor Audiences Only GD: Phase 2 trial initiation GD: initial results at 12 and 24 weeks MG: topline results 2023 2024 2025 MG: Phase 3 Trial Initiation TED: Initiation of Phase 3 trials TED: topline results for both trials CIDP: initial results from open label period CIDP: Pivotal 2b trial initiation Target Indication Stage of Development Myasthenia Gravis (MG) Pivotal Phase 3 Thyroid Eye Disease (TED) Pivotal Phase 3 Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Pivotal Phase 2b* Graves' Disease (GD) Phase 2 Warm Autoimmune Hemolytic Anemia (WAIHA) Phase 2** Data Trial Initiation 2022 2026 Planning for regular cadence of data across indications

Pete Salzmann, MD Chief Executive Officer Bill Macias, MD Chief Medical Officer Investor Q&A