
Immunovant R&D Day Enabling normal lives for people with autoimmune disease March 30, 2022 Exhibit 99.1

Forward-looking statements 2 This presentation contains forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. The use of words such as “may,” “might,” “will,” “would,” “should,” “expect,” “believe,” “estimate,” “design,” “plan,” and other similar expressions are intended to identify forward-looking statements. Such forward looking statements include Immunovant’s plan to start a Phase 3 study for batoclimab in myasthenia gravis (MG) in the first half of calendar year 2022 with an expected data readout in 2024, and expectations with respect to the safety and monitoring plan and size of the safety database; Immunovant’s plan to explore in subsequent study periods follow-on treatment with alternative dosing regimens; Immunovant’s plan to develop batoclimab across a broad range of autoimmune indications; Immunovant’s expectations regarding timing, the design and results of clinical trials of its product candidates and indication selections; Immunovant's beliefs regarding its cash runway, and the potential benefits of batoclimab’s unique product attributes. All forward-looking statements are based on estimates and assumptions by Immunovant’s management that, although Immunovant believes to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that Immunovant expected. Such risks and uncertainties include, among others: initial results or other preliminary analyses or results of early clinical trials may not be predictive final trial results or of the results of later clinical trials; the timing and availability of data from clinical trials; the timing of discussions with regulatory agencies, as well as regulatory submissions and potential approvals; the continued development of Immunovant’s product candidate, including the timing of the commencement of additional clinical trials and resumption of current trials; Immunovant’s scientific approach, clinical trial design, indication selection and general development progress; future clinical trials may not confirm any safety, potency or other product characteristics described or assumed in this presentation; any product candidate that Immunovant develops may not progress through clinical development or receive required regulatory approvals within expected timelines or at all; Immunovant’s product candidate may not be beneficial to patients, or even if approved by regulatory authorities, successfully commercialized; the potential impact of the ongoing COVID-19 pandemic on Immunovant’s clinical development plans and timelines; Immunovant’s business is heavily dependent on the successful development, regulatory approval and commercialization of its sole product candidate, batoclimab; Immunovant is at an early stage in development of batoclimab; and Immunovant will require additional capital to fund its operations and advance batoclimab through clinical development. These and other risks and uncertainties are more fully described in Immunovant’s periodic and other reports filed with the Securities and Exchange Commission (SEC), including in the section titled “Risk Factors” in Immunovant’s most recent Annual Report on Form 10-K, its Form 10-Q filed with the SEC on February 4, 2022, and Immunovant’s subsequent filings with the SEC. Any forward-looking statement speaks only as of the date on which it was made. Immunovant undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. All trademarks, trade names, service marks, and copyrights appearing in this presentation are the property of their respective owners.

Q&A housekeeping 3 You may submit questions through the Q&A function at the bottom of the webcast player. Please see the image here for reference. For Investor Audiences Only

Today’s agenda 4 Immunovant and batoclimab vision and strategy | Pete Salzmann, MD, CEO Immunovant Thyroid Eye Disease an exciting opportunity | Bill Macias, MD, CMO Immunovant • Andrea Kossler1, MD, FACS, Stanford University School of Medicine • George Kahaly2, MD, PhD, Johannes Gutenberg University Medical Center • Pete Salzmann, MD, CEO Immunovant Myasthenia Gravis a multifaceted disease | Pete Salzmann, MD, CEO Immunovant • Katherine Ruzhansky3, MD, MS, Medical University of South Carolina • Nicholas Silvestri4, MD, FAAN, University of Buffalo Warm Autoimmune Hemolytic Anemia opportunity for innovative treatment options | Pete Salzmann, MD, CEO Immunovant • David Tucker5 MB ChB, BSc, MD MRCP, FRCPath, Royal Cornwall NHS Hospitals Trust Cholesterol management what we know | Bill Macias, MD, CMO Immunovant • Michael Davidson6, MD, University of Chicago, Pritzker School of Medicine Path forward what comes next | Pete Salzmann, MD, CEO Immunovant Q&A Financial Disclosures : 1. Consultant, Horizon & Immunovant; Research Funds Horizontal Pharmaceuticals, Viridian Pharmaceuticals, VasaraGen Inc. 2. The Johannes Gutenberg University (JGU) Medical Center, Mainz, Germany (academic institution of George J Kahaly, MD, PhD) has received research-associated funding from the JGU Medical Faculty, AdvanceCor (Germany), Apitope (Belgium), Berlin-Chemie (Germany), Byondis (The Netherlands), GlycoEra (Sw itzerland), Horizon (USA), Immunovant (USA), ISAR (Germany), Mediomics (USA), Merck (Germany), Novartis (USA), Quidel (USA), River Vision (USA), and Roche (Sw itzerland). GJK consults for GlycoEra, Immunovant, ISAR, Mediomics, Merck, Novartis, Quidel, & VasaraGen (USA). 3. Consultant/advisory board for: Alexion, Argenx, Ra/UCB, Immunovant; Current grant/research funding from: Alexion, Ra/UCB, Janssen, Myasthenia Gravis Foundation of America, MGNet 4. medical advisory boards and speaker for argenx, UCB, advisory boards for Immunovant, Alexion, Biogen, Roche, speaker for Strongbridge/Xeris 5. Advisory board honoraria: Roche, Abbvie, Novartis, Consultant, Immunovant 6. Consultant, Immunovant For Investor Audiences Only

Broad development plan enabled by an exciting mechanism of action, batoclimab’s features and a strong balance sheet 5 01 02 03 Immunovant remains on track to start three pivotal studies in calendar year 20223, and to announce two new indications by August 2022 1. As of December 31, 2021, per most recent Quarterly Report on Form 10-Q filed w ith the SEC on February 4, 2022 2. The assumptions upon w hich we have based our estimates are routinely evaluated and may be subject to change 3. Three pivotal trials include myasthenia gravis and tw o other indications Batoclimab's unique combination of attributes present a potentially significant commercial opportunity across multiple indications in rare autoimmune diseases Immunovant is well-capitalized with $527M1 in cash expected to fund its broad development plans into calendar year 20252 5 04 Immunovant plans to initiate a Phase 3 study of batoclimab in Myasthenia Gravis in the first half of calendar year 2022 – topline results expected in 2024 For Investor Audiences Only

Pete Salzmann, MD Chief Executive Officer Vision and Strategy
Our vision: Normal lives for people with autoimmune disease Driven by our core values 7 Love Trailblazing All Voices Bolder, Faster For Investor Audiences Only
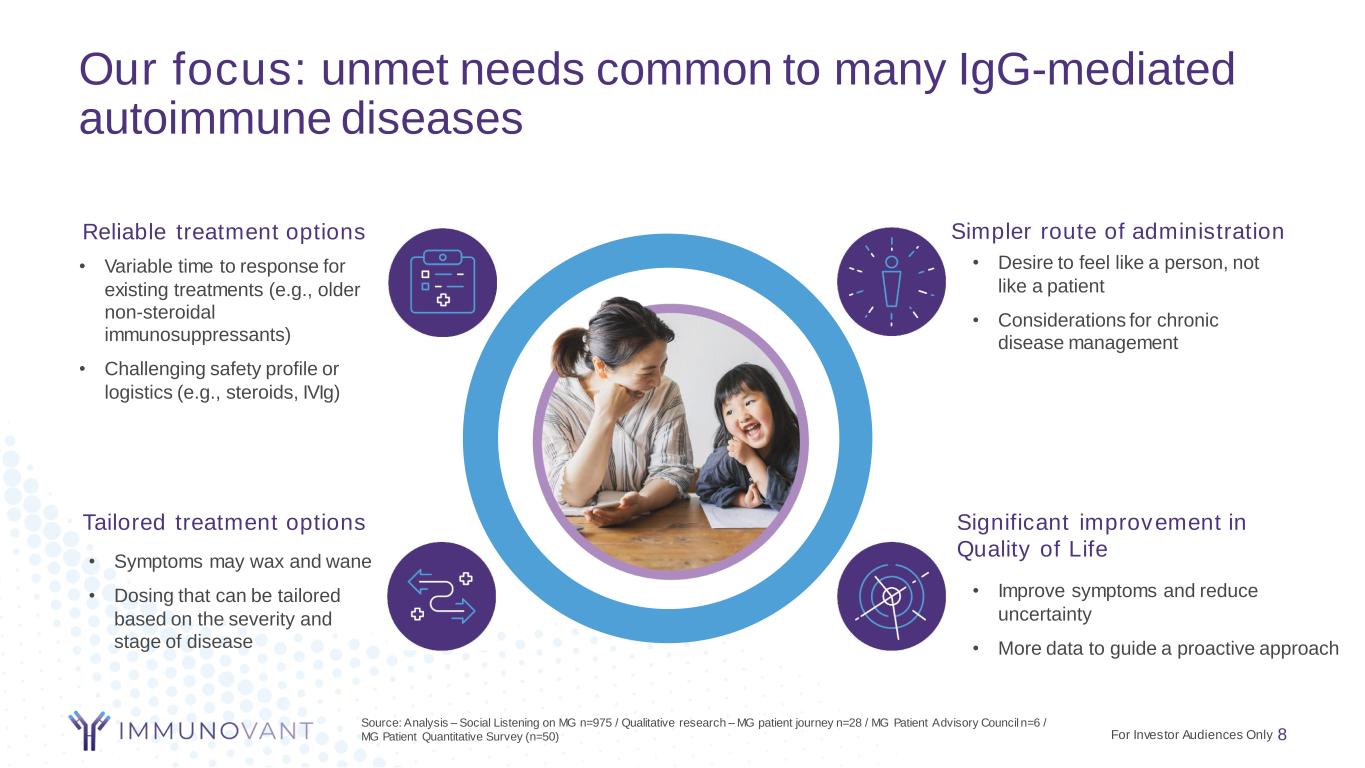
Our focus: unmet needs common to many IgG-mediated autoimmune diseases 8 • Improve symptoms and reduce uncertainty • More data to guide a proactive approach Significant improvement in Quality of Life • Variable time to response for existing treatments (e.g., older non-steroidal immunosuppressants) • Challenging safety profile or logistics (e.g., steroids, IVIg) Reliable treatment options • Symptoms may wax and wane • Dosing that can be tailored based on the severity and stage of disease Tailored treatment options • Desire to feel like a person, not like a patient • Considerations for chronic disease management Simpler route of administration Source: Analysis – Social Listening on MG n=975 / Qualitative research – MG patient journey n=28 / MG Patient Advisory Council n=6 / MG Patient Quantitative Survey (n=50) For Investor Audiences Only
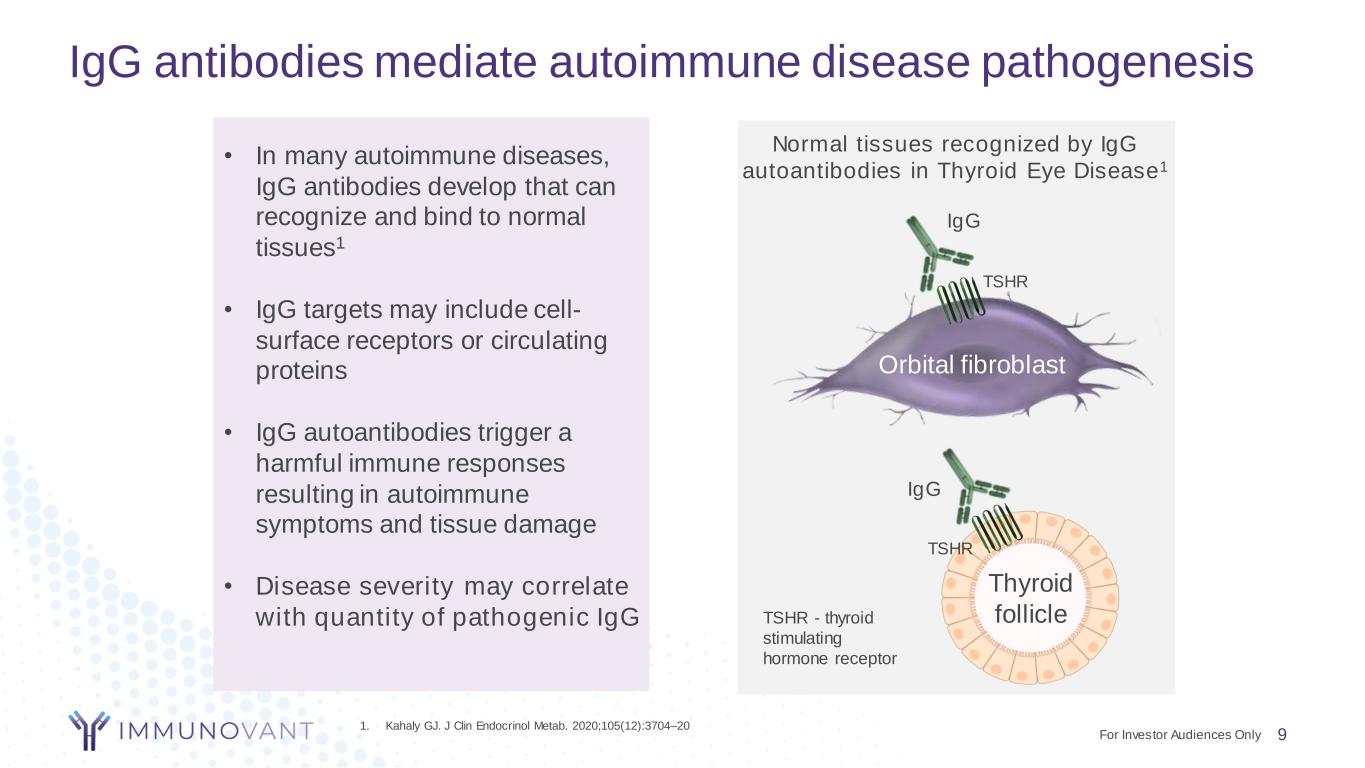
IgG antibodies mediate autoimmune disease pathogenesis • In many autoimmune diseases, IgG antibodies develop that can recognize and bind to normal tissues1 • IgG targets may include cell- surface receptors or circulating proteins • IgG autoantibodies trigger a harmful immune responses resulting in autoimmune symptoms and tissue damage • Disease severity may correlate with quantity of pathogenic IgG Thyroid follicle Orbital fibroblast TSHR IgG Normal tissues recognized by IgG autoantibodies in Thyroid Eye Disease1 TSHR IgG 1. Kahaly GJ. J Clin Endocrinol Metab. 2020;105(12):3704–20 TSHR - thyroid stimulating hormone receptor 9For Investor Audiences Only
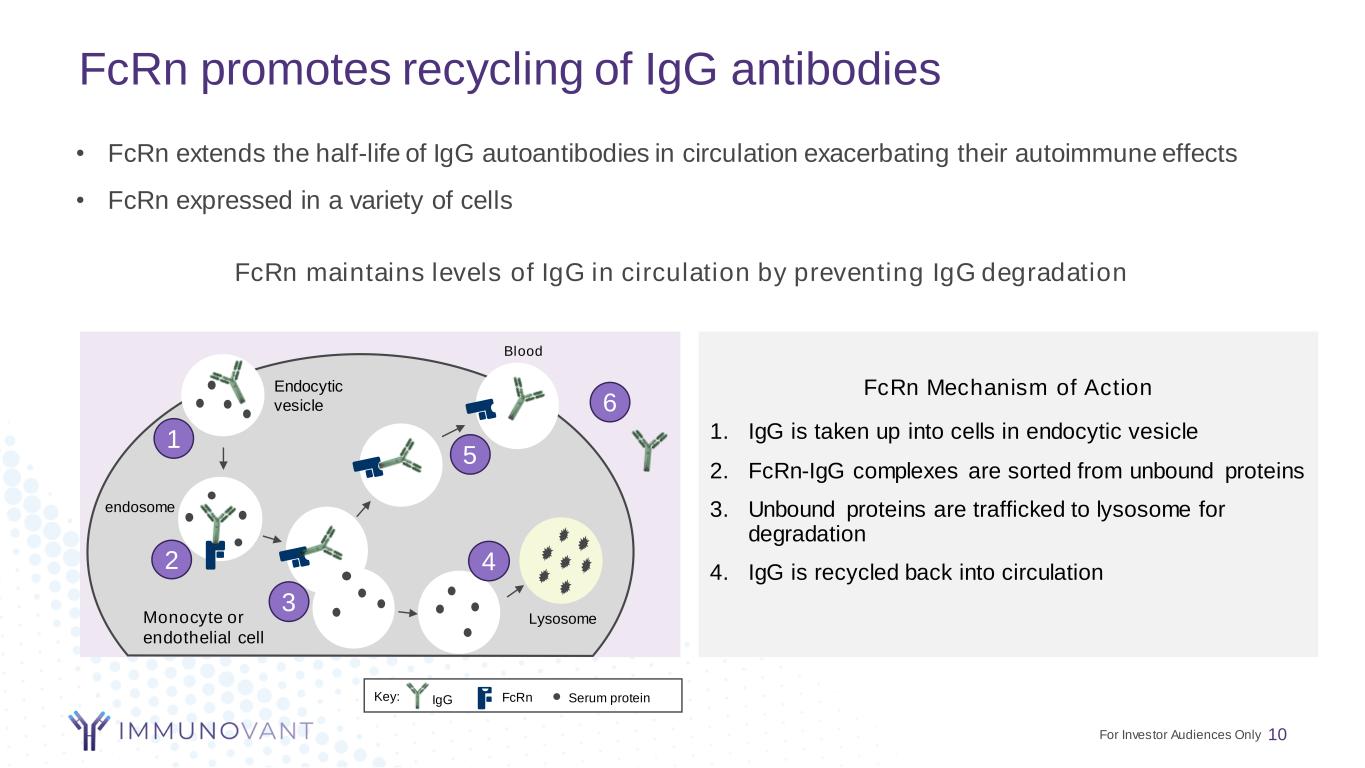
FcRn promotes recycling of IgG antibodies 10 FcRn Mechanism of Action 1. IgG is taken up into cells in endocytic vesicle 2. FcRn-IgG complexes are sorted from unbound proteins 3. Unbound proteins are trafficked to lysosome for degradation 4. IgG is recycled back into circulation FcRn maintains levels of IgG in circulation by preventing IgG degradation • FcRn extends the half-life of IgG autoantibodies in circulation exacerbating their autoimmune effects • FcRn expressed in a variety of cells Blood Endocytic vesicle endosome LysosomeMonocyte or endothelial cell Key: Serum proteinIgG FcRn 1 2 3 4 5 6 For Investor Audiences Only
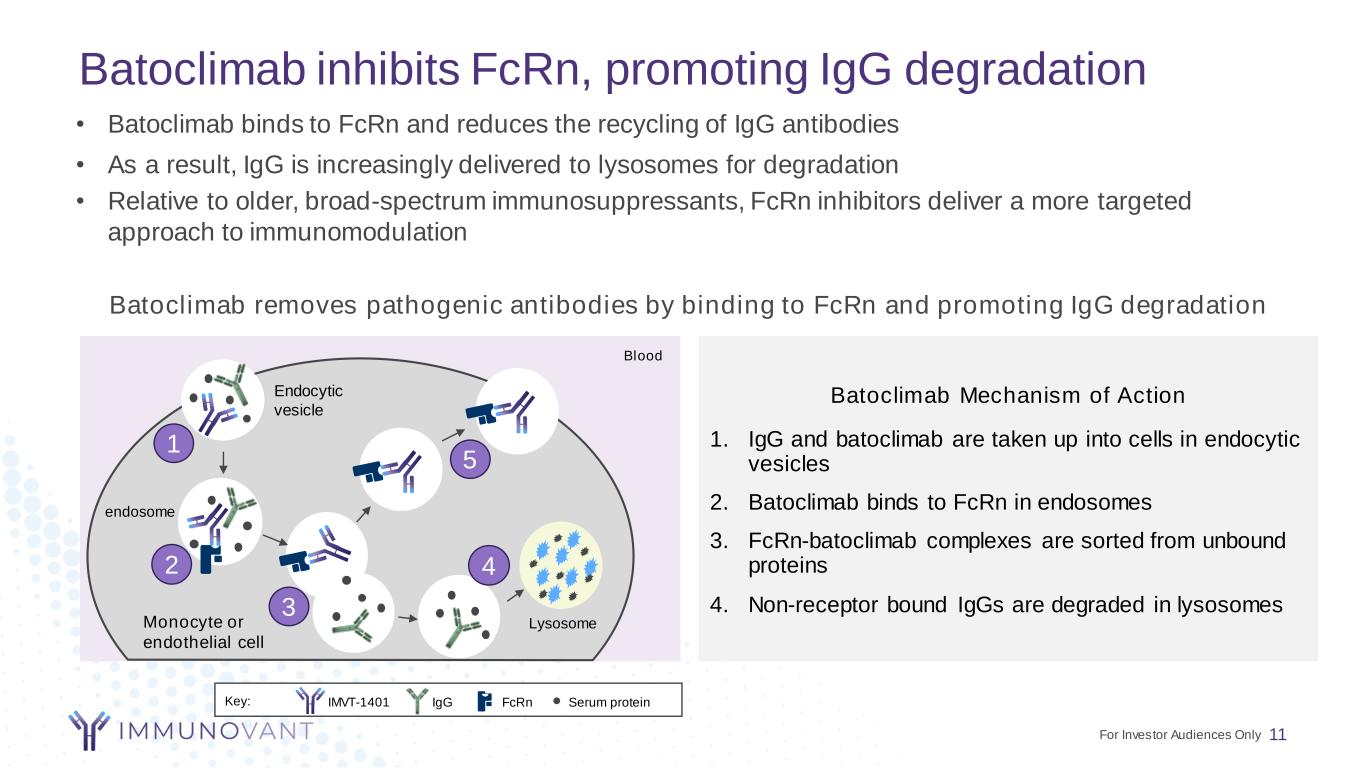
Batoclimab inhibits FcRn, promoting IgG degradation 11 Batoclimab Mechanism of Action 1. IgG and batoclimab are taken up into cells in endocytic vesicles 2. Batoclimab binds to FcRn in endosomes 3. FcRn-batoclimab complexes are sorted from unbound proteins 4. Non-receptor bound IgGs are degraded in lysosomes Batoclimab removes pathogenic antibodies by binding to FcRn and promoting IgG degradation • Batoclimab binds to FcRn and reduces the recycling of IgG antibodies • As a result, IgG is increasingly delivered to lysosomes for degradation • Relative to older, broad-spectrum immunosuppressants, FcRn inhibitors deliver a more targeted approach to immunomodulation Blood Endocytic vesicle endosome LysosomeMonocyte or endothelial cell Key: Serum proteinIgG FcRn 1 2 3 4 5 IMVT-1401 For Investor Audiences Only
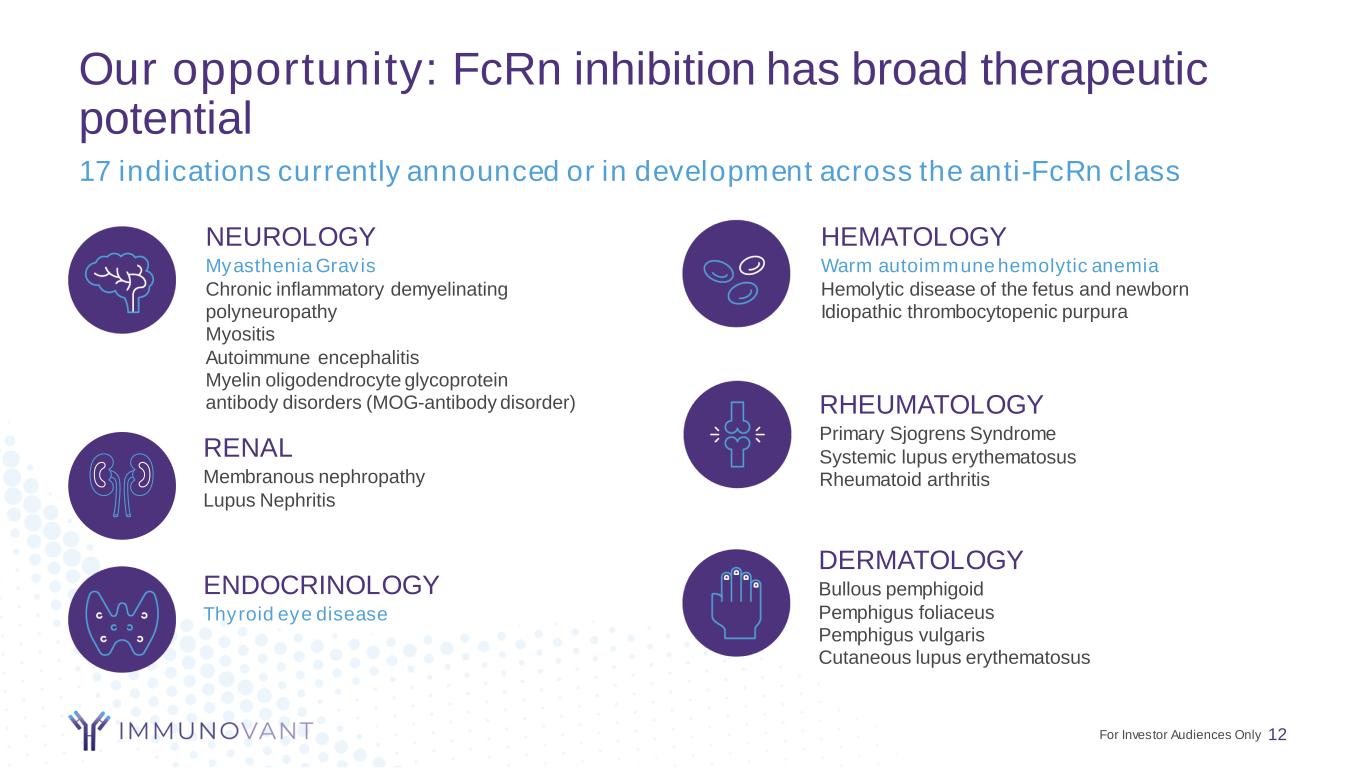
Our opportunity: FcRn inhibition has broad therapeutic potential 12 17 indications currently announced or in development across the anti-FcRn class ENDOCRINOLOGY Thyroid eye disease RHEUMATOLOGY Primary Sjogrens Syndrome Systemic lupus erythematosus Rheumatoid arthritis NEUROLOGY Myasthenia Gravis Chronic inflammatory demyelinating polyneuropathy Myositis Autoimmune encephalitis Myelin oligodendrocyte glycoprotein antibody disorders (MOG-antibody disorder) HEMATOLOGY Warm autoimmune hemolytic anemia Hemolytic disease of the fetus and newborn Idiopathic thrombocytopenic purpura For Investor Audiences Only DERMATOLOGY Bullous pemphigoid Pemphigus foliaceus Pemphigus vulgaris Cutaneous lupus erythematosus RENAL Membranous nephropathy Lupus Nephritis
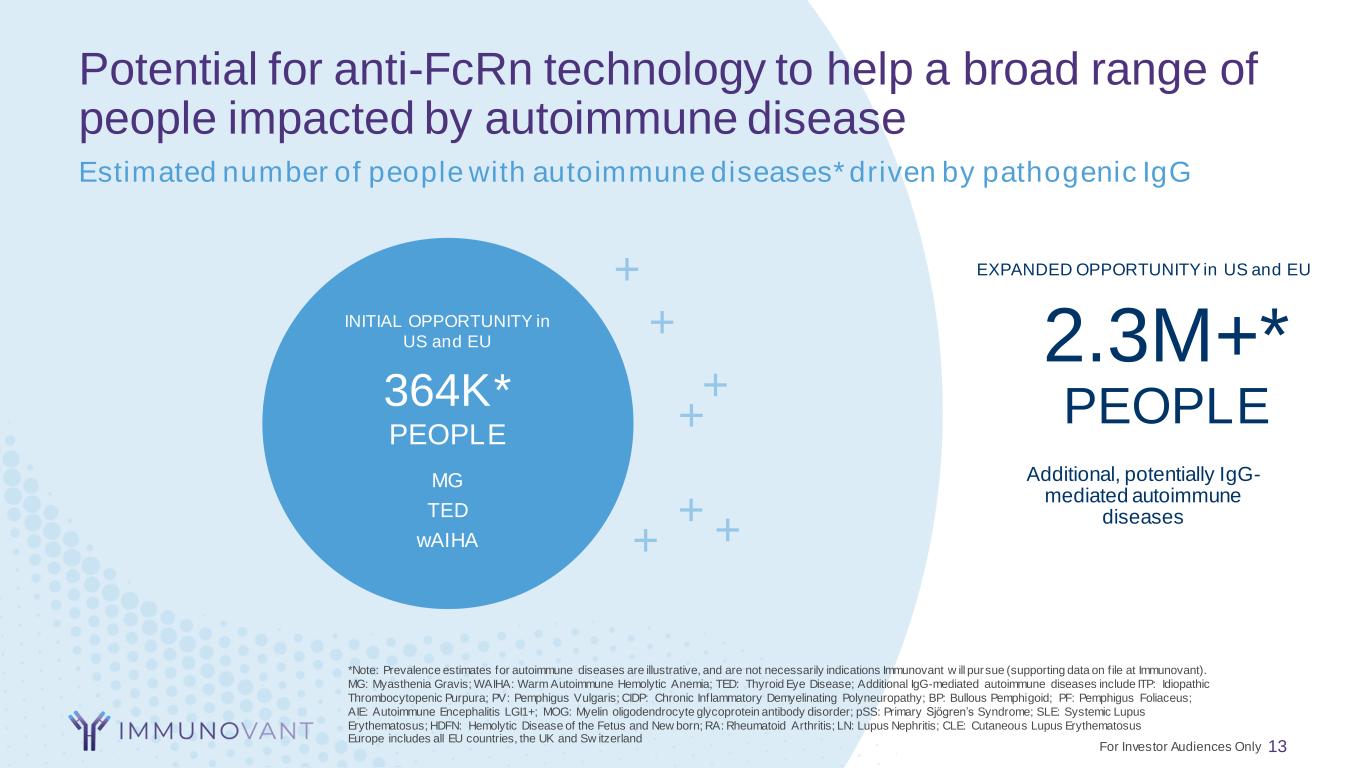
Potential for anti-FcRn technology to help a broad range of people impacted by autoimmune disease 13 2.3M+* PEOPLE Additional, potentially IgG- mediated autoimmune diseases EXPANDED OPPORTUNITY in US and EU INITIAL OPPORTUNITY in US and EU 364K* PEOPLE MG TED wAIHA Estimated number of people with autoimmune diseases* driven by pathogenic IgG + + + + + + + *Note: Prevalence estimates for autoimmune diseases are illustrative, and are not necessarily indications Immunovant w ill pursue (supporting data on f ile at Immunovant). MG: Myasthenia Gravis; WAIHA: Warm Autoimmune Hemolytic Anemia; TED: Thyroid Eye Disease; Additional IgG-mediated autoimmune diseases include ITP: Idiopathic Thrombocytopenic Purpura; PV: Pemphigus Vulgaris; CIDP: Chronic Inflammatory Demyelinating Polyneuropathy; BP: Bullous Pemphigoid; PF: Pemphigus Foliaceus; AIE: Autoimmune Encephalitis LGI1+; MOG: Myelin oligodendrocyte glycoprotein antibody disorder; pSS: Primary Sjögren’s Syndrome; SLE: Systemic Lupus Erythematosus; HDFN: Hemolytic Disease of the Fetus and New born; RA: Rheumatoid Arthritis; LN: Lupus Nephritis; CLE: Cutaneous Lupus Erythematosus Europe includes all EU countries, the UK and Sw itzerland For Investor Audiences Only
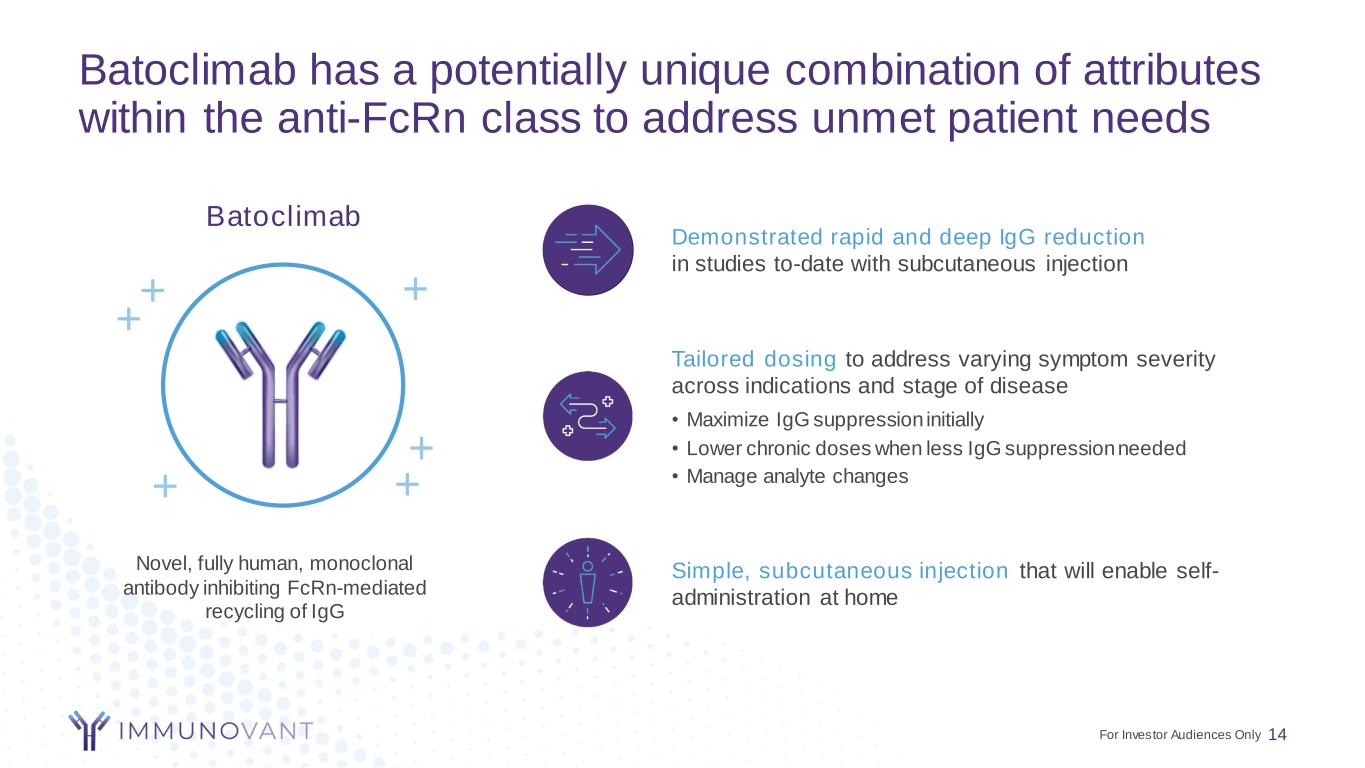
Batoclimab has a potentially unique combination of attributes within the anti-FcRn class to address unmet patient needs 14 Novel, fully human, monoclonal antibody inhibiting FcRn-mediated recycling of IgG Batoclimab Tailored dosing to address varying symptom severity across indications and stage of disease • Maximize IgG suppression initially • Lower chronic doses when less IgG suppression needed • Manage analyte changes Simple, subcutaneous injection that will enable self- administration at home Demonstrated rapid and deep IgG reduction in studies to-date with subcutaneous injection ++ + + + + For Investor Audiences Only
Pioneering anti-FcRn technology to meaningfully advance the quality of care for people living with autoimmune diseases 15 Novel, fully human, monoclonal antibody inhibiting FcRn-mediated recycling of IgG Batoclimab + + + + + + Intentionally designed to deliver potent IgG reductions via a standard sub-cutaneous injection for tailored & sustained disease control + For Investor Audiences Only
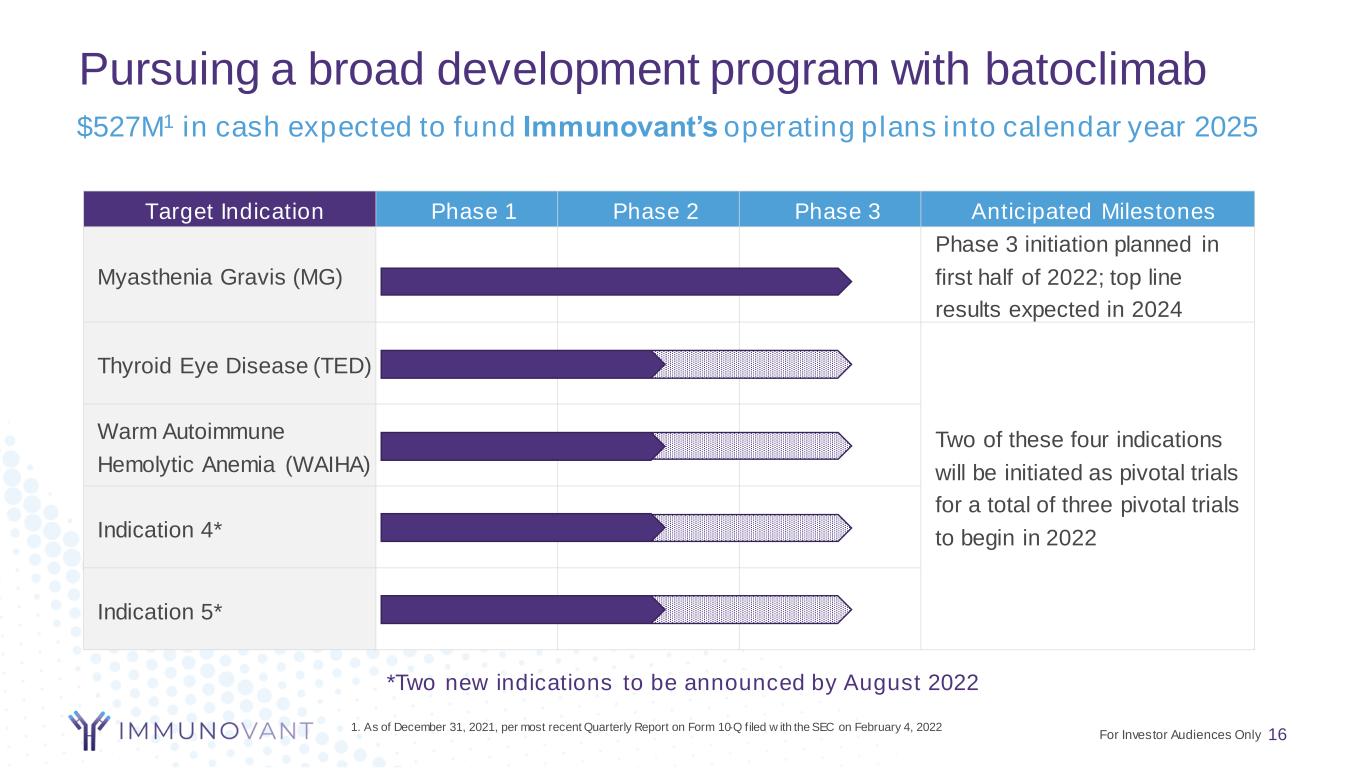
Pursuing a broad development program with batoclimab 16 Target Indication Phase 1 Phase 2 Phase 3 Anticipated Milestones Myasthenia Gravis (MG) Phase 3 initiation planned in first half of 2022; top line results expected in 2024 Thyroid Eye Disease (TED) Two of these four indications will be initiated as pivotal trials for a total of three pivotal trials to begin in 2022 Warm Autoimmune Hemolytic Anemia (WAIHA) Indication 4* Indication 5* *Two new indications to be announced by August 2022 $527M1 in cash expected to fund Immunovant’s operating plans into calendar year 2025 1. As of December 31, 2021, per most recent Quarterly Report on Form 10-Q filed w ith the SEC on February 4, 2022 For Investor Audiences Only

Pete Salzmann, MD Chief Executive Officer Thyroid Eye Disease An exciting opportunity Pre-Record

18

Opportunities in Thyroid Eye Disease LIVE
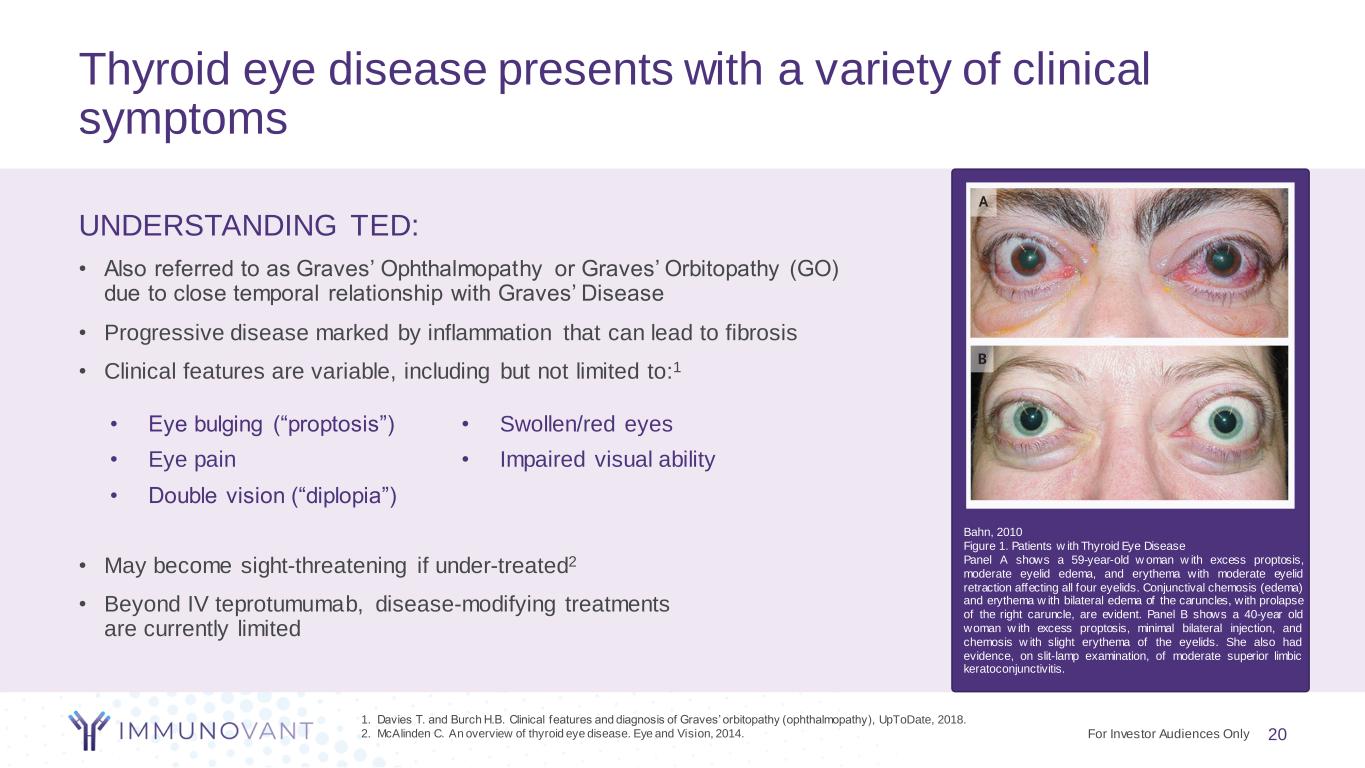
Thyroid eye disease presents with a variety of clinical symptoms UNDERSTANDING TED: • Also referred to as Graves’ Ophthalmopathy or Graves’ Orbitopathy (GO) due to close temporal relationship with Graves’ Disease • Progressive disease marked by inflammation that can lead to fibrosis • Clinical features are variable, including but not limited to:1 • May become sight-threatening if under-treated2 • Beyond IV teprotumumab, disease-modifying treatments are currently limited 20 1. Davies T. and Burch H.B. Clinical features and diagnosis of Graves’ orbitopathy (ophthalmopathy), UpToDate, 2018. 2. McAlinden C. An overview of thyroid eye disease. Eye and Vision, 2014. • Eye bulging (“proptosis”) • Eye pain • Double vision (“diplopia”) • Swollen/red eyes • Impaired visual ability Bahn, 2010 Figure 1. Patients w ith Thyroid Eye Disease Panel A shows a 59-year-old w oman w ith excess proptosis, moderate eyelid edema, and erythema with moderate eyelid retraction affecting all four eyelids. Conjunctival chemosis (edema) and erythema w ith bilateral edema of the caruncles, with prolapse of the right caruncle, are evident. Panel B shows a 40-year old woman w ith excess proptosis, minimal bilateral injection, and chemosis w ith slight erythema of the eyelids. She also had evidence, on slit-lamp examination, of moderate superior limbic keratoconjunctivitis. For Investor Audiences Only
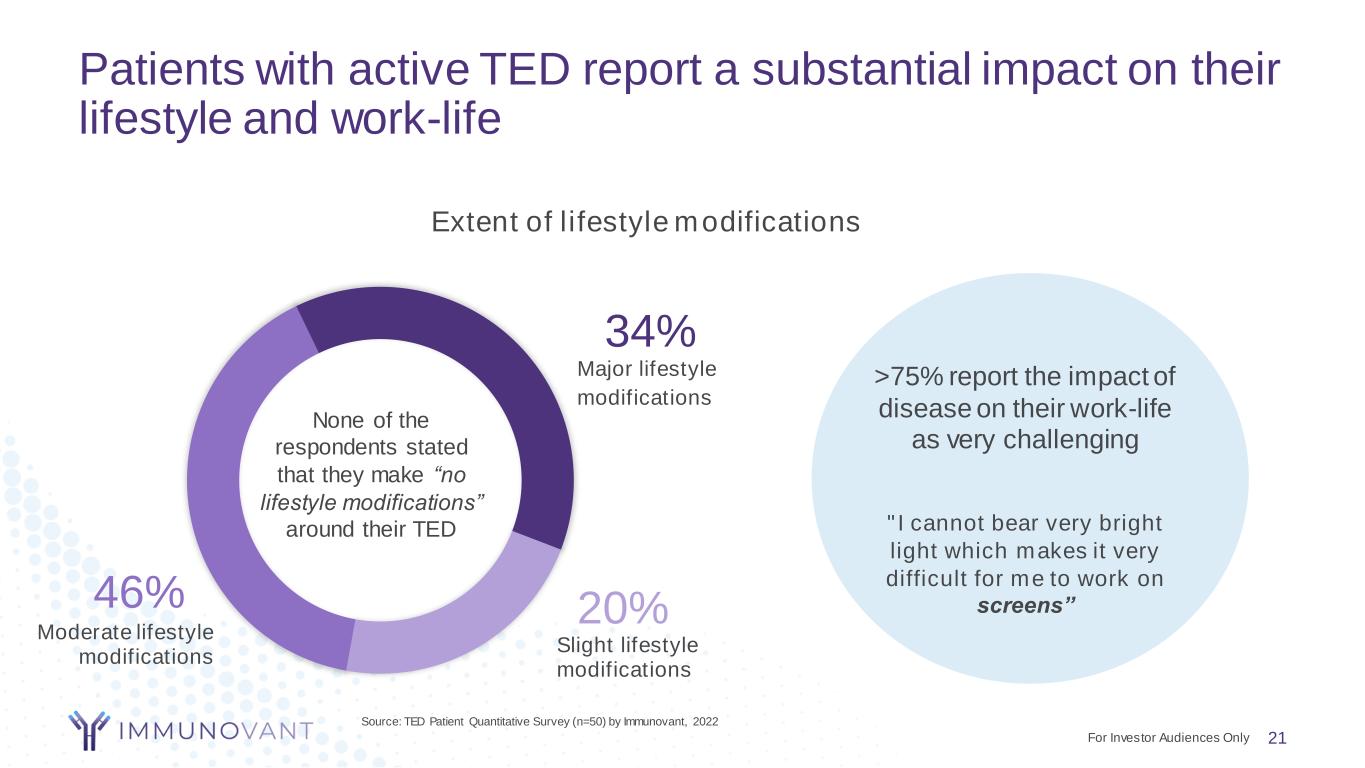
Patients with active TED report a substantial impact on their lifestyle and work-life 21 Source: TED Patient Quantitative Survey (n=50) by Immunovant, 2022 Moderate lifestyle modifications 46% None of the respondents stated that they make “no lifestyle modifications” around their TED Major lifestyle modifications 34% Slight lifestyle modifications 20% Extent of lifestyle modifications >75% report the impact of disease on their work-life as very challenging "I cannot bear very bright light which makes it very difficult for me to work on screens” For Investor Audiences Only
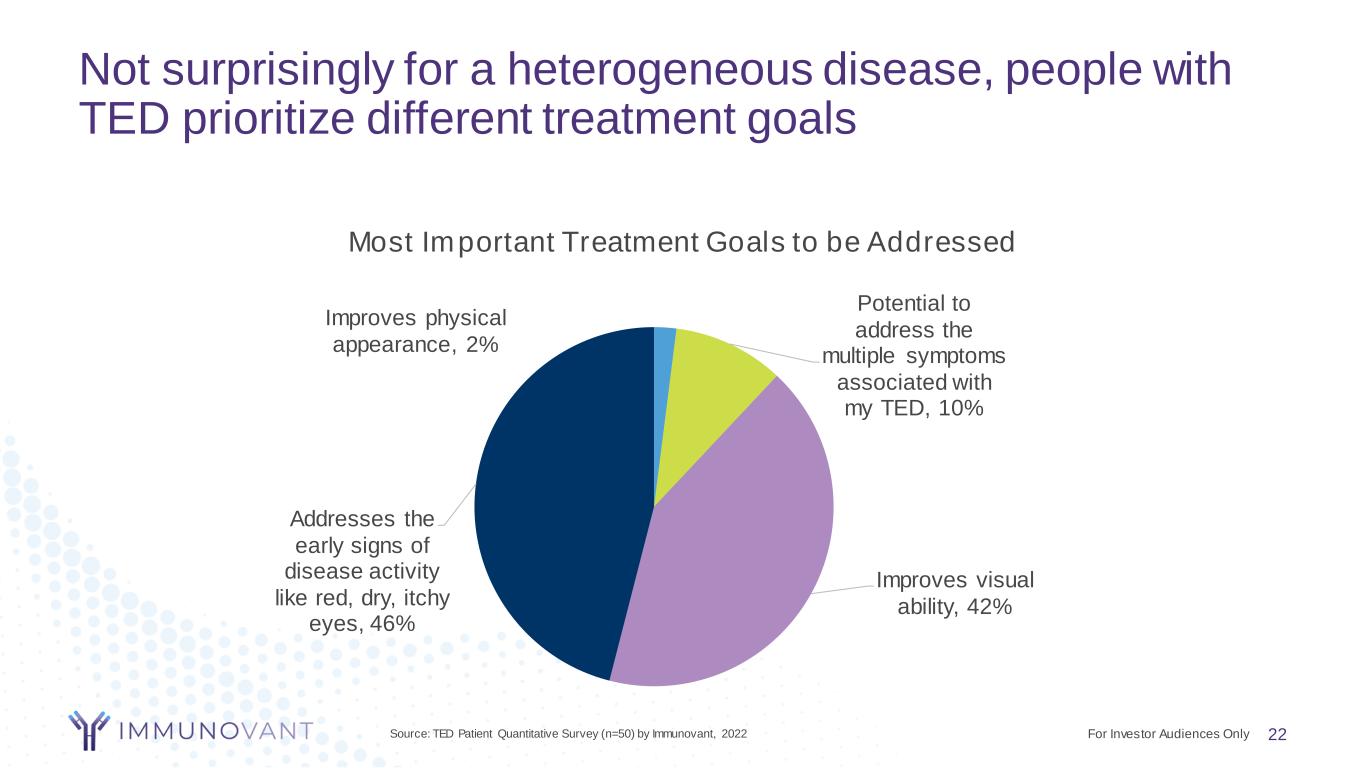
Not surprisingly for a heterogeneous disease, people with TED prioritize different treatment goals 22Source: TED Patient Quantitative Survey (n=50) by Immunovant, 2022 Improves physical appearance, 2% Potential to address the multiple symptoms associated with my TED, 10% Improves visual ability, 42% Addresses the early signs of disease activity like red, dry, itchy eyes, 46% Most Important Treatment Goals to be Addressed For Investor Audiences Only

23

Understanding Thyroid Eye Disease
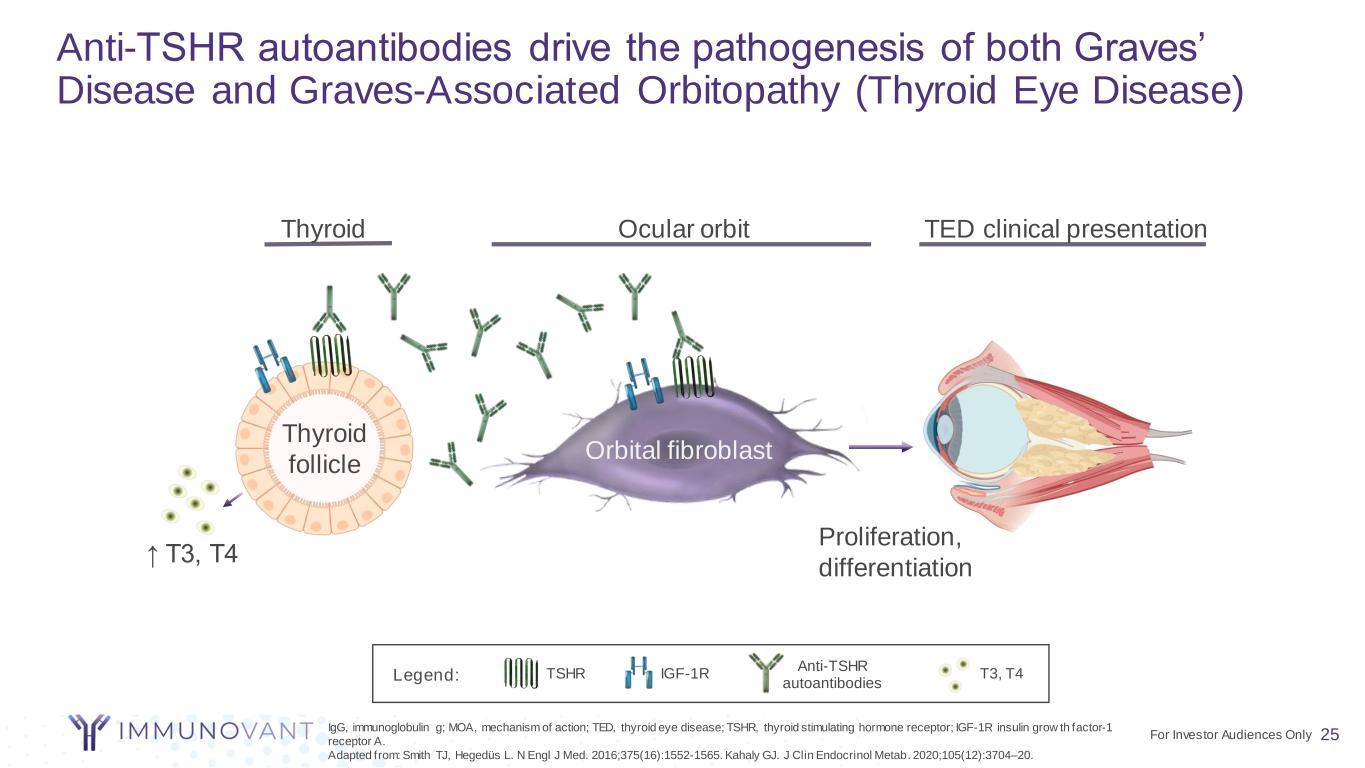
25 TSHR IGF-1R Anti-TSHR autoantibodies TED clinical presentationOcular orbitThyroid Thyroid follicle Orbital fibroblast Proliferation, differentiation IgG, immunoglobulin g; MOA, mechanism of action; TED, thyroid eye disease; TSHR, thyroid stimulating hormone receptor; IGF-1R insulin grow th factor-1 receptor A. Adapted from: Smith TJ, Hegedüs L. N Engl J Med. 2016;375(16):1552-1565. Kahaly GJ. J Clin Endocrinol Metab. 2020;105(12):3704–20. ↑ T3, T4 Anti-TSHR autoantibodies drive the pathogenesis of both Graves’ Disease and Graves-Associated Orbitopathy (Thyroid Eye Disease) T3, T4Legend: For Investor Audiences Only
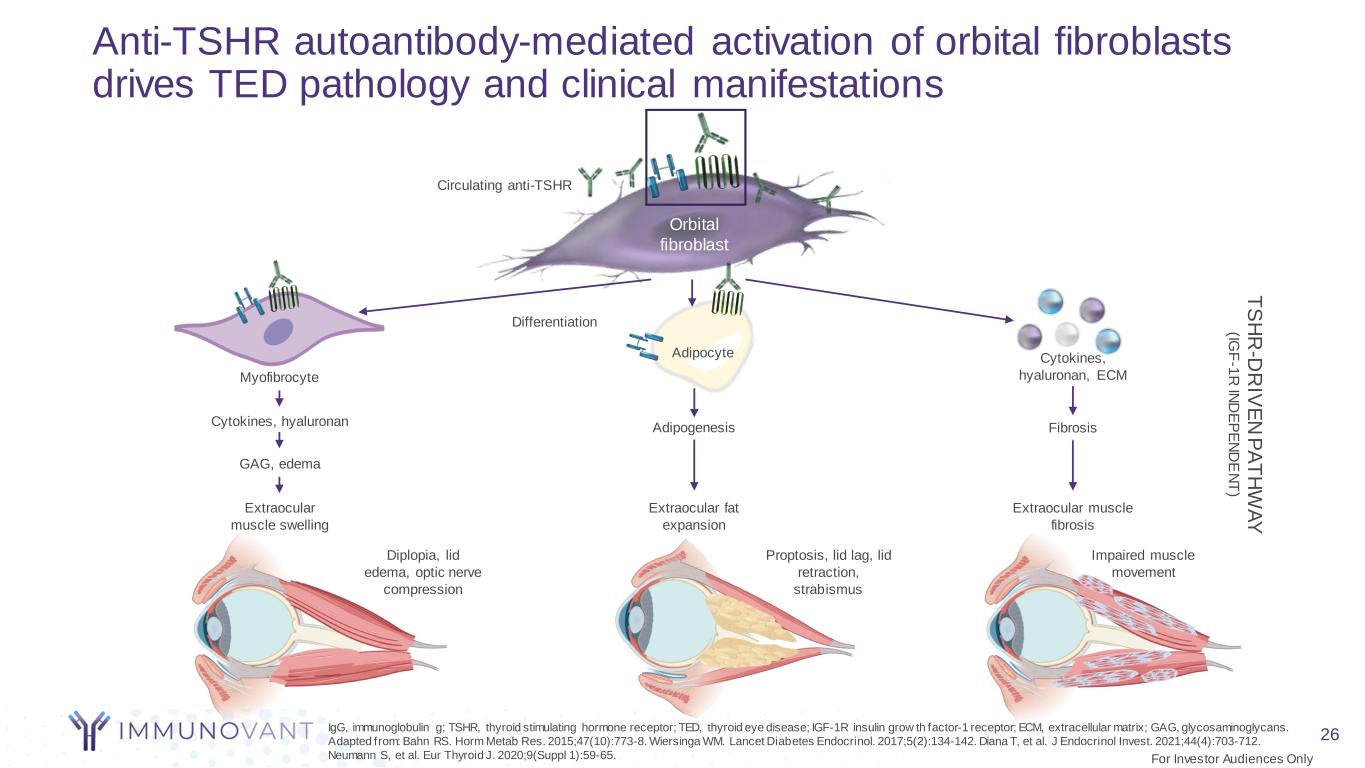
26 Anti-TSHR autoantibody-mediated activation of orbital fibroblasts drives TED pathology and clinical manifestations GAG, edema Diplopia, lid edema, optic nerve compression Differentiation Adipocyte Orbital fibroblast T S H R -D R IV E N P A T H W A Y (IG F -1 R IN D E P E N D E N T ) Cytokines, hyaluronan Extraocular muscle swelling Adipogenesis Extraocular fat expansion Extraocular muscle fibrosis Impaired muscle movement Circulating anti-TSHR Myofibrocyte Fibrosis Cytokines, hyaluronan, ECM Proptosis, lid lag, lid retraction, strabismus IgG, immunoglobulin g; TSHR, thyroid stimulating hormone receptor; TED, thyroid eye disease; IGF-1R insulin grow th factor-1 receptor; ECM, extracellular matrix; GAG, glycosaminoglycans. Adapted from: Bahn RS. Horm Metab Res. 2015;47(10):773-8. Wiersinga WM. Lancet Diabetes Endocrinol. 2017;5(2):134-142. Diana T, et al. J Endocrinol Invest. 2021;44(4):703-712. Neumann S, et al. Eur Thyroid J. 2020;9(Suppl 1):59-65. For Investor Audiences Only
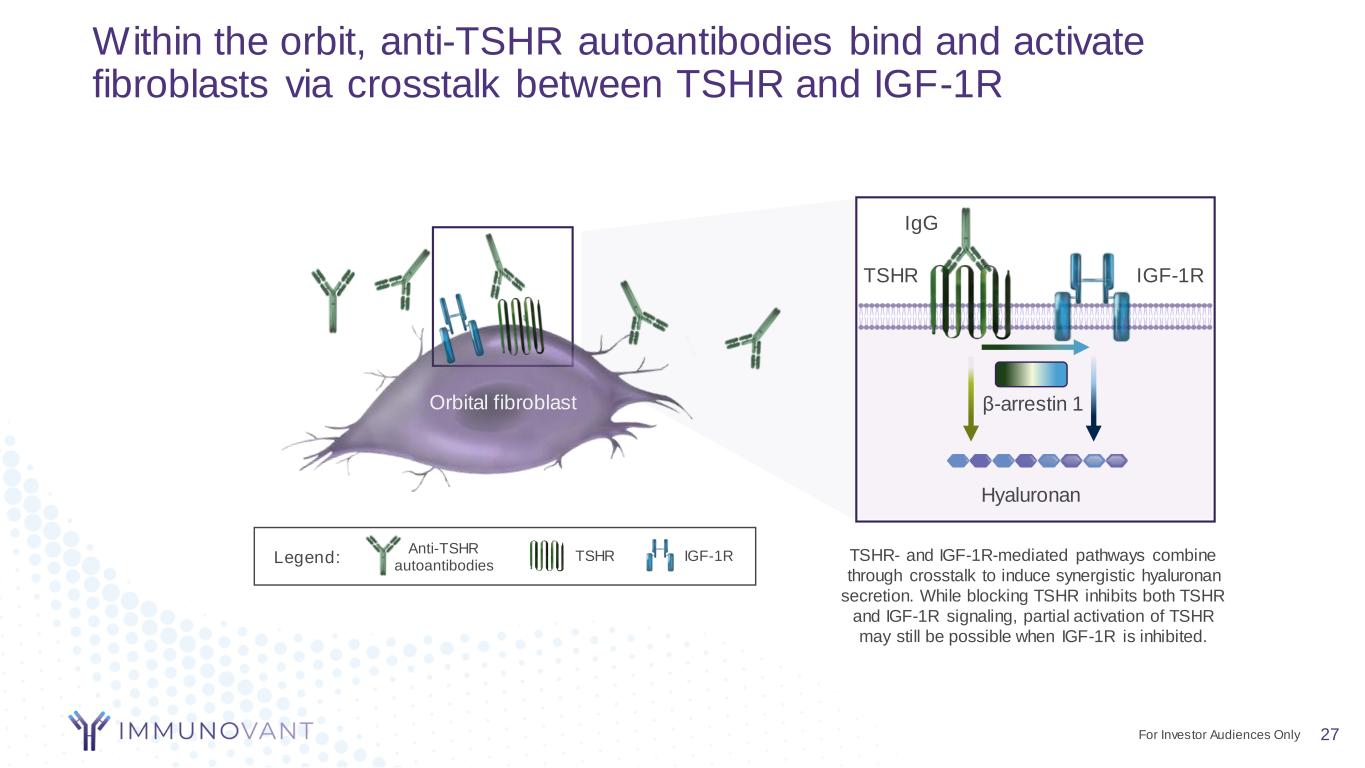
27 Within the orbit, anti-TSHR autoantibodies bind and activate fibroblasts via crosstalk between TSHR and IGF-1R Orbital fibroblast IgG TSHR IGF-1R β-arrestin 1 Hyaluronan TSHR- and IGF-1R-mediated pathways combine through crosstalk to induce synergistic hyaluronan secretion. While blocking TSHR inhibits both TSHR and IGF-1R signaling, partial activation of TSHR may still be possible when IGF-1R is inhibited. TSHR IGF-1R Anti-TSHR autoantibodies Legend: For Investor Audiences Only
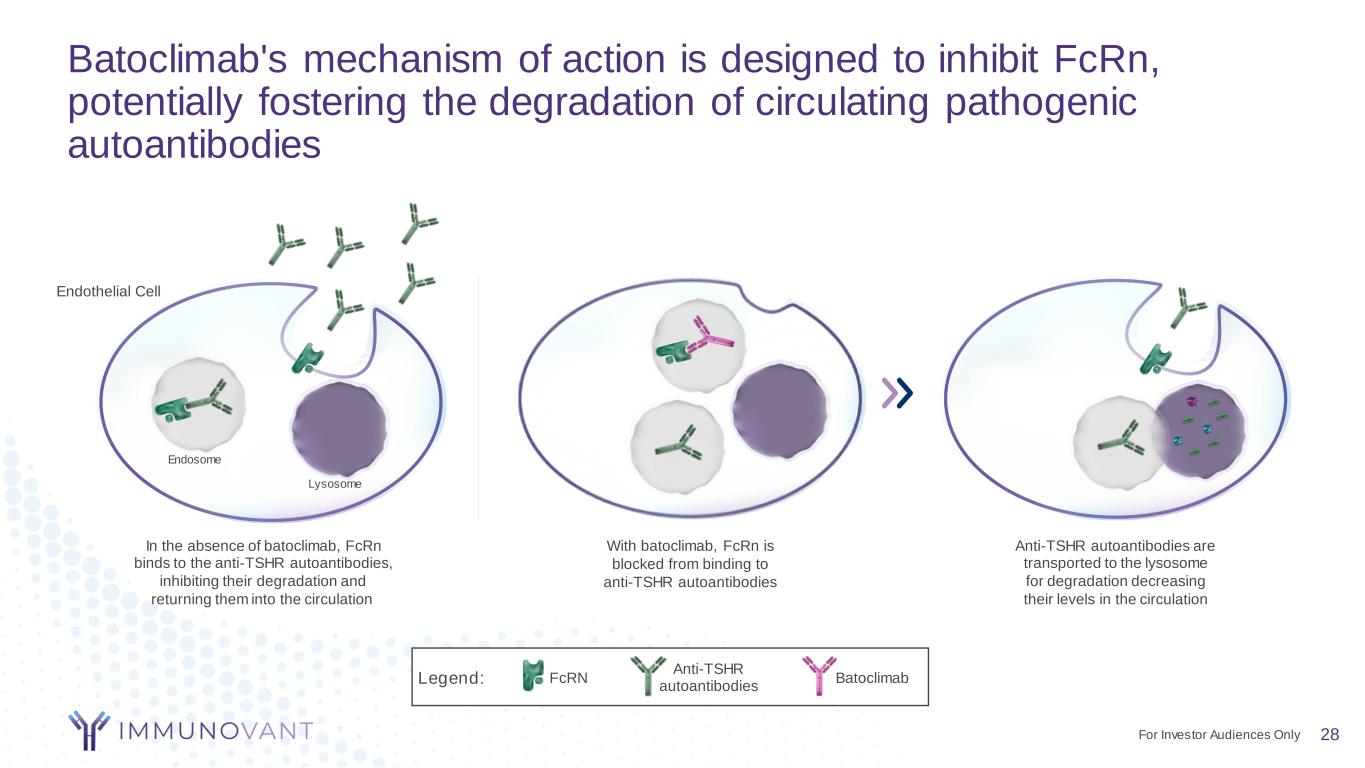
Batoclimab's mechanism of action is designed to inhibit FcRn, potentially fostering the degradation of circulating pathogenic autoantibodies 28 Anti-TSHR autoantibodies are transported to the lysosome for degradation decreasing their levels in the circulation With batoclimab, FcRn is blocked from binding to anti-TSHR autoantibodies In the absence of batoclimab, FcRn binds to the anti-TSHR autoantibodies, inhibiting their degradation and returning them into the circulation Anti-TSHR autoantibodies BatoclimabFcRNLegend: Endothelial Cell Endosome Lysosome For Investor Audiences Only
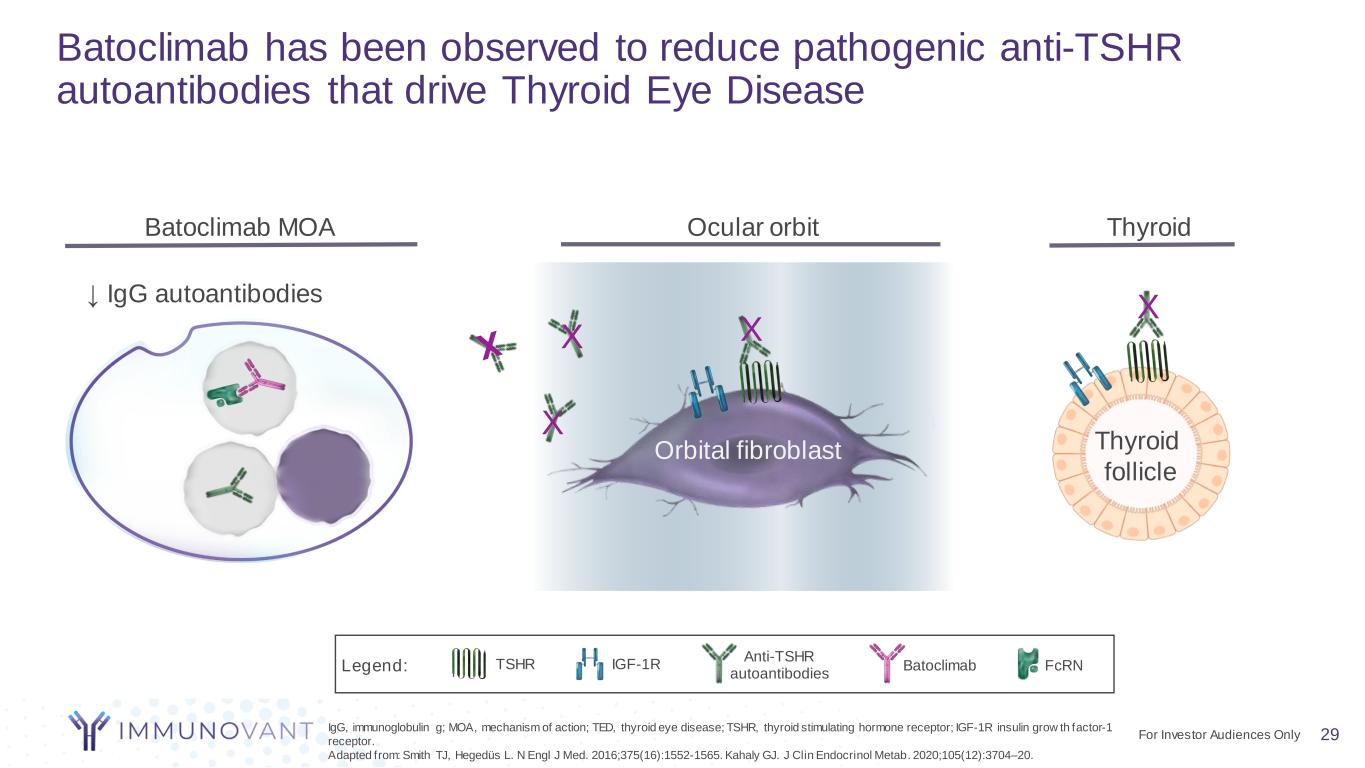
29 Batoclimab has been observed to reduce pathogenic anti-TSHR autoantibodies that drive Thyroid Eye Disease Ocular orbit Thyroid Orbital fibroblast X X Batoclimab MOA ↓ IgG autoantibodies IgG, immunoglobulin g; MOA, mechanism of action; TED, thyroid eye disease; TSHR, thyroid stimulating hormone receptor; IGF-1R insulin grow th factor-1 receptor. Adapted from: Smith TJ, Hegedüs L. N Engl J Med. 2016;375(16):1552-1565. Kahaly GJ. J Clin Endocrinol Metab. 2020;105(12):3704–20. Thyroid follicle X Anti-TSHR autoantibodies Batoclimab FcRNTSHR IGF-1RLegend: X For Investor Audiences Only

Opportunities in Thyroid Eye Disease LIVE

Batoclimab in Thyroid Eye Disease Exploratory analyses used to inform further development because trial voluntarily halted with inconclusive primary endpoint
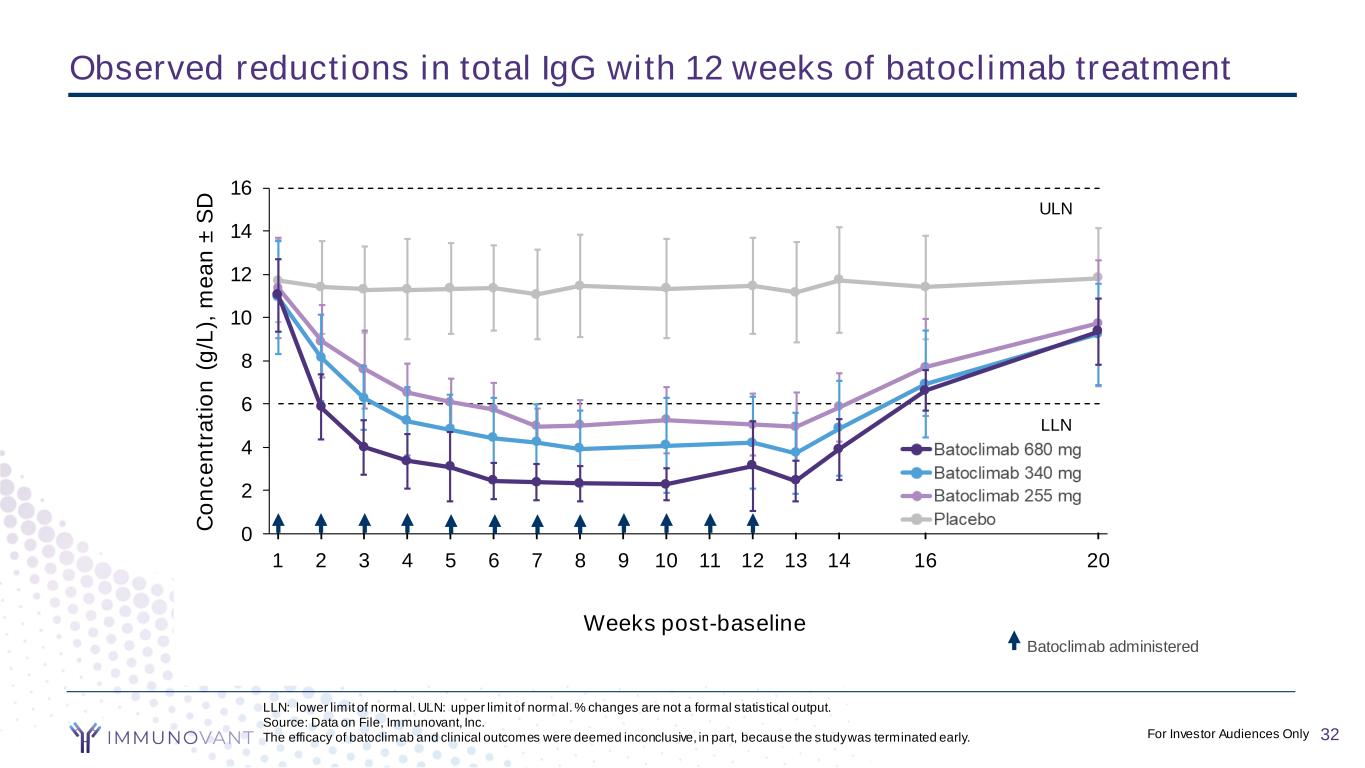
CONFIDENTIAL 32 0 1 2 3 4 5 6 7 8 9 10 11 12 13 15 19 13 13 13 13 13 13 12 13 13 12 12 12 12 12 6 6 6 6 6 6 4 6 6 6 5 6 6 6 14 13 14 14 13 12 12 12 12 13 12 12 13 12 12 12 12 12 12 11 9 11 11 12 10 11 10 10 ULN LLN 0 2 4 6 8 10 12 14 16 C o n c e n tr a ti o n ( g /L ), m e a n ± S D Weeks post-baseline Batoclimab 680 mg Batoclimab 340 mg Batoclimab 255 mg Placebo 1 2 3 4 5 6 7 8 9 10 1 2 3 4 6 20 C o n c e n tr a ti o n ( g /L ), m e a n ± S D Weeks post-baseline Batoclimab 340 mg Batoclimab 255 mg Placebo LLN: lower limit of normal. ULN: upper limit of normal. % changes are not a formal statistical output. Source: Data on File, Immunovant, Inc. The efficacy of batoclimab and clinical outcomes were deemed inconclusive, in part, because the study was terminated early. Observed reductions in total IgG with 12 weeks of batoclimab treatment 32For Investor Audiences Only Batoclimab administered
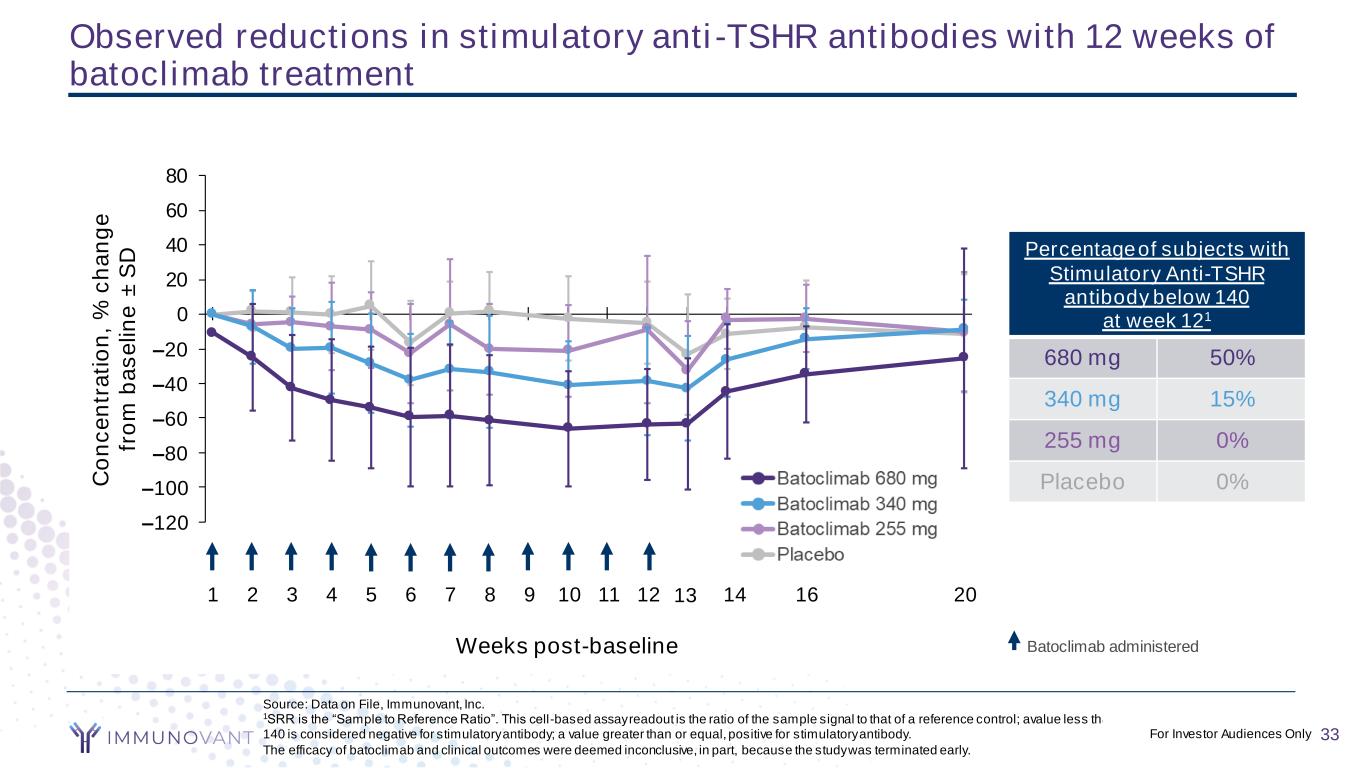
CONFIDENTIAL 33 Source: Data on File, Immunovant, Inc. 1SRR is the “Sample to Reference Ratio”. This cell-based assay readout is the ratio of the sample signal to that of a reference control; avalue less than 140 is considered negative for stimulatory antibody; a value greater than or equal, positive for stimulatory antibody. The efficacy of batoclimab and clinical outcomes were deemed inconclusive, in part, because the study was terminated early. Observed reductions in stimulatory anti-TSHR antibodies with 12 weeks of batoclimab treatment 0 1 2 3 4 5 6 7 8 9 10 11 12 13 15 19 18 18 17 16 16 16 16 16 16 14 12 12 11 13 10 10 9 9 9 9 9 9 8 7 6 6 6 6 19 17 18 16 17 17 16 16 14 13 13 12 13 12 18 17 17 14 13 13 13 12 12 11 10 10 9 9 –120 –80 –40 0 40 80 Title C o n c e n tr a ti o n , % c h a n g e fr o m b a s e li n e ± S D Title Batoclimab 680 mg Batoclimab 340 mg Batoclimab 255 mg Placebo 1 2 3 4 5 6 7 8 9 10 11 12 13 14 16 20 –100 –60 –20 20 60 C o n c e n tr a ti o n , % c h a n g e fr o m b a s e li n e ± S D Batoclimab 340 mg Batoclimab 255 mg Placebo Percentage of subjects with Stimulatory Anti-TSHR antibody below 140 at week 121 680 mg 50% 340 mg 15% 255 mg 0% Placebo 0% Weeks post-baseline 33For Investor Audiences Only Batoclimab administered
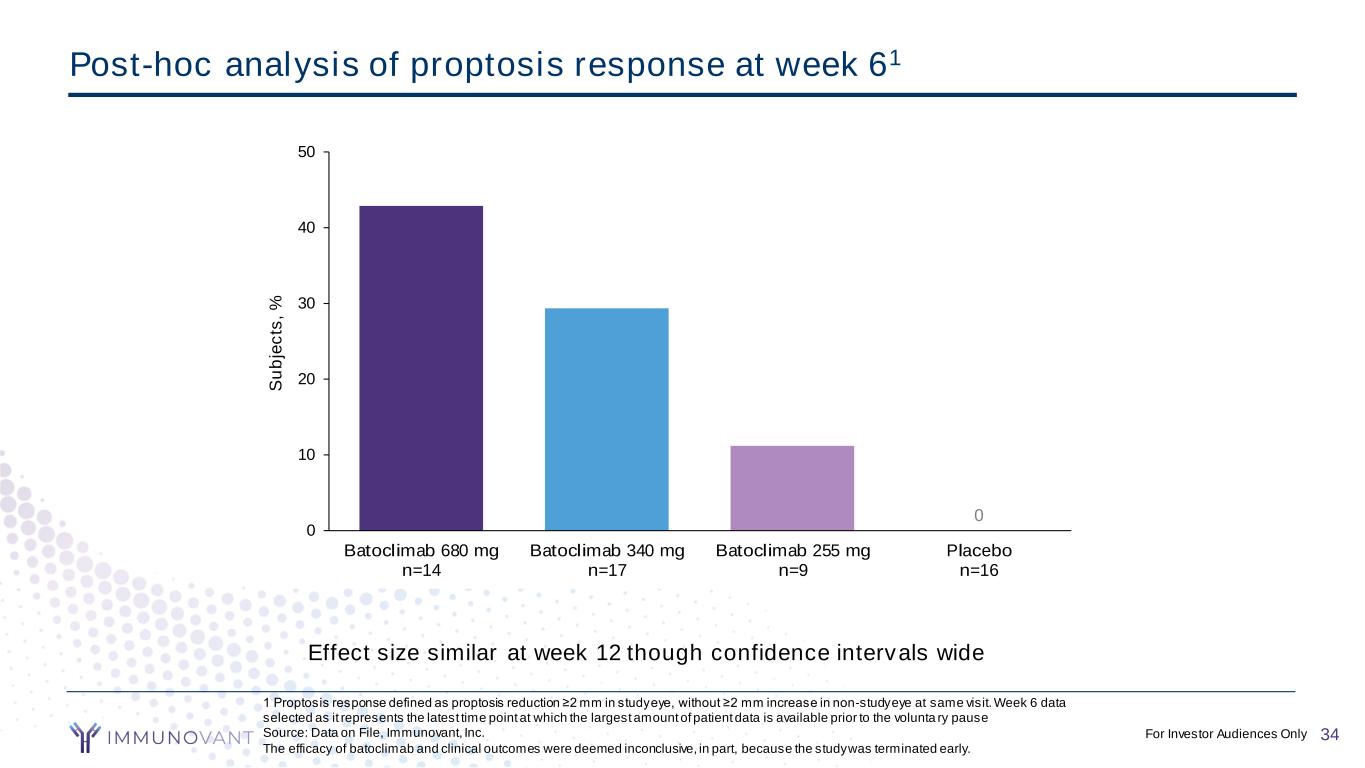
CONFIDENTIAL 1 Proptosis response defined as proptosis reduction ≥2 mm in study eye, without ≥2 mm increase in non-study eye at same visit. Week 6 data selected as it represents the latest time point at which the largest amount of patient data is available prior to the volunta ry pause Source: Data on File, Immunovant, Inc. The efficacy of batoclimab and clinical outcomes were deemed inconclusive, in part, because the study was terminated early. Post-hoc analysis of proptosis response at week 61 0 0 10 20 30 40 50 Batoclimab 680 mg n=14 Batoclimab 340 mg n=17 Batoclimab 255 mg n=9 Placebo n=16 S u b je c ts , % Effect size similar at week 12 though confidence intervals wide 34For Investor Audiences Only
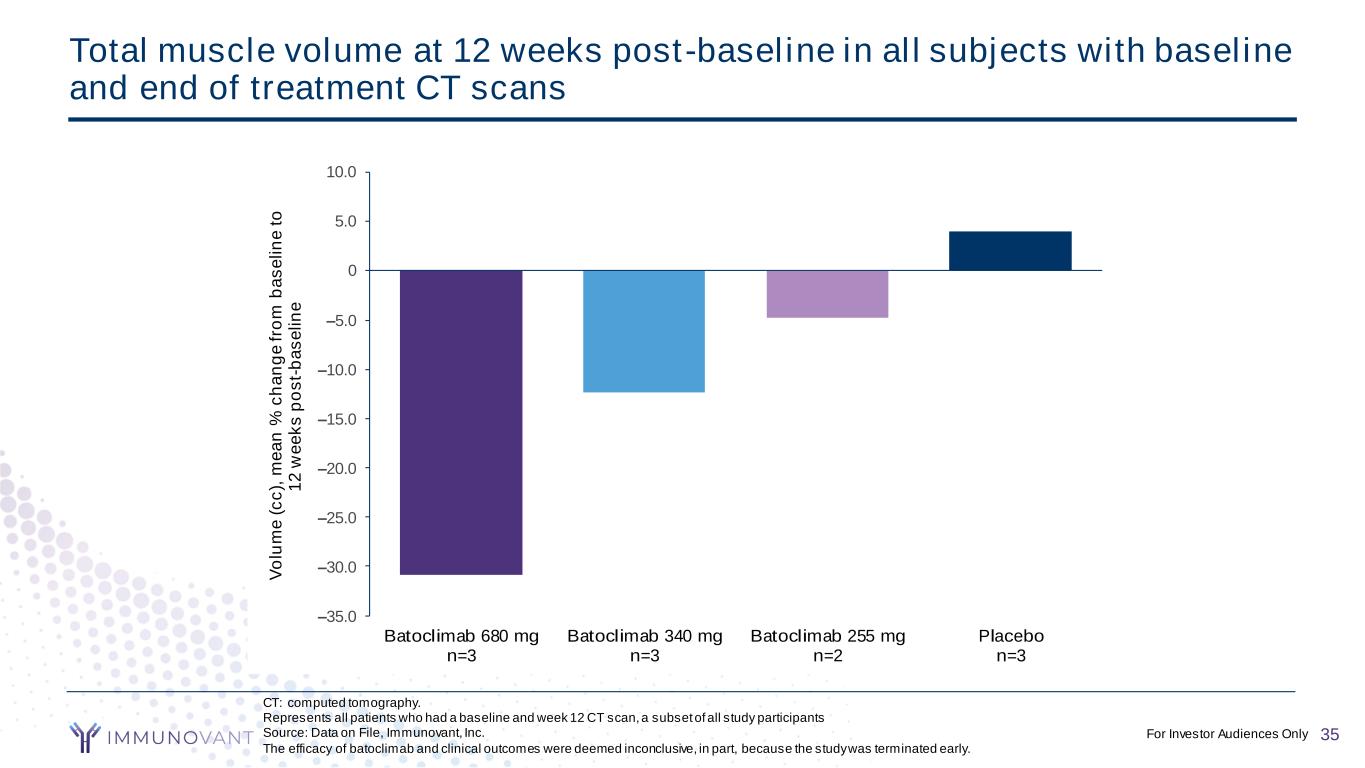
CONFIDENTIAL CT: computed tomography. Represents all patients who had a baseline and week 12 CT scan, a subset of all study participants Source: Data on File, Immunovant, Inc. The efficacy of batoclimab and clinical outcomes were deemed inconclusive, in part, because the study was terminated early. Total muscle volume at 12 weeks post-baseline in all subjects with baseline and end of treatment CT scans –35.0 –30.0 –25.0 –20.0 –15.0 –10.0 –5.0 0 5.0 10.0 Batoclimab 680 mg n=3 Batoclimab 340 mg n=3 Batoclimab 255 mg n=2 Placebo n=3 V o lu m e ( c c ), m e a n % c h a n g e f ro m b a s e li n e t o 1 2 w e e k s p o s t- b a s e li n e 35For Investor Audiences Only
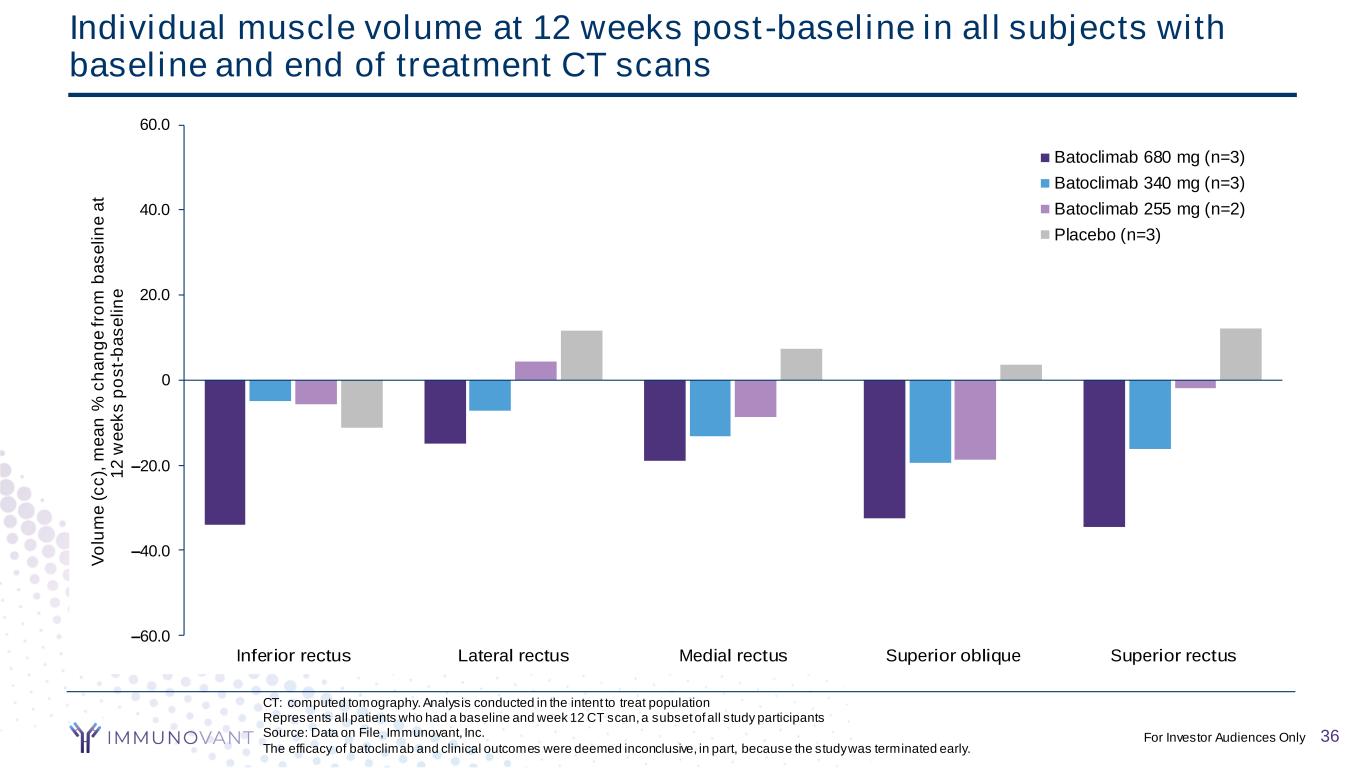
CONFIDENTIAL CT: computed tomography. Analysis conducted in the intent to treat population Represents all patients who had a baseline and week 12 CT scan, a subset of all study participants Source: Data on File, Immunovant, Inc. The efficacy of batoclimab and clinical outcomes were deemed inconclusive, in part, because the study was terminated early. Individual muscle volume at 12 weeks post-baseline in all subjects with baseline and end of treatment CT scans –60.0 –40.0 –20.0 0 20.0 40.0 60.0 Inferior rectus Lateral rectus Medial rectus Superior oblique Superior rectus V o lu m e ( c c ), m e a n % c h a n g e f ro m b a s e li n e a t 1 2 w e e k s p o s t- b a s e li n e Batoclimab 680 mg (n=3) Batoclimab 340 mg (n=3) Batoclimab 255 mg (n=2) Placebo (n=3) 36For Investor Audiences Only

Thyroid Eye Disease An exciting opportunity
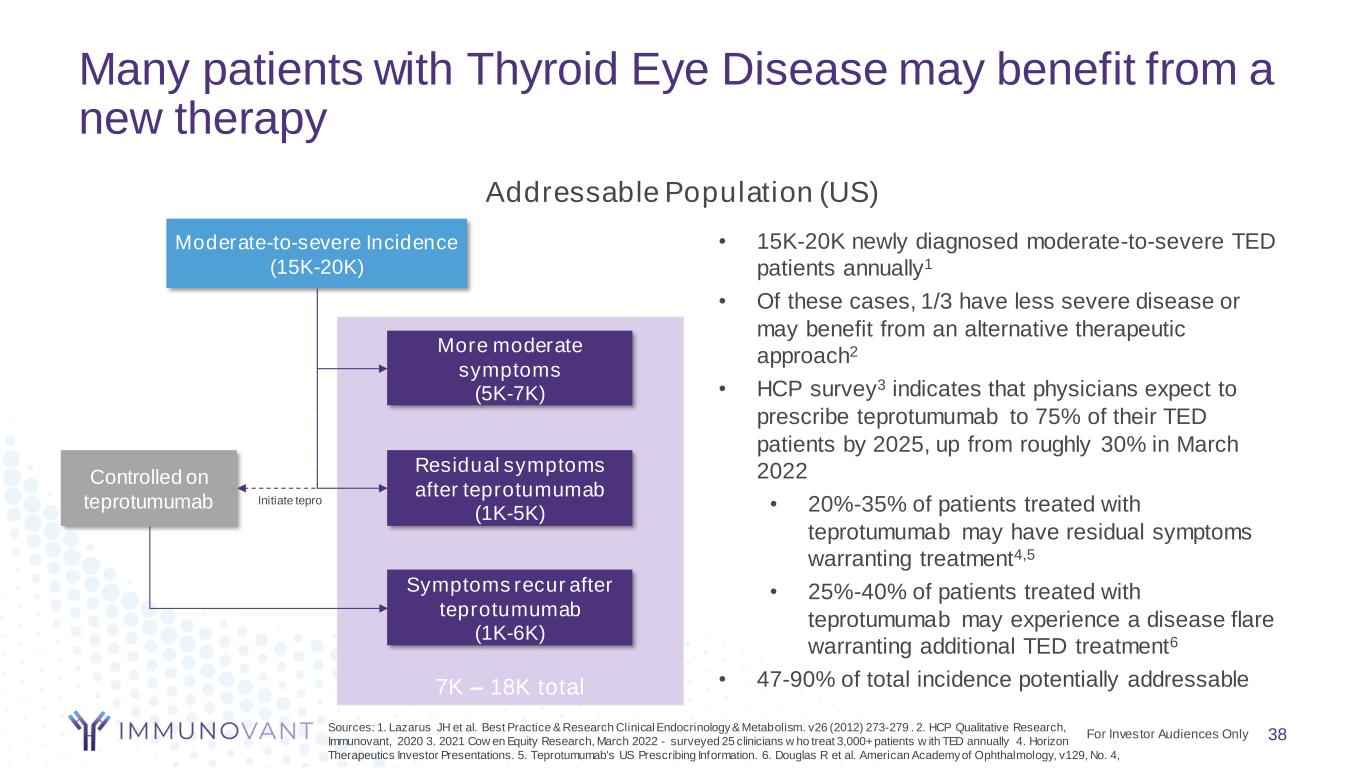
7K – 18K total Many patients with Thyroid Eye Disease may benefit from a new therapy 38 Addressable Population (US) Moderate-to-severe Incidence (15K-20K) Residual symptoms after teprotumumab (1K-5K) Controlled on teprotumumab Symptoms recur after teprotumumab (1K-6K) Initiate tepro More moderate symptoms (5K-7K) • 15K-20K newly diagnosed moderate-to-severe TED patients annually1 • Of these cases, 1/3 have less severe disease or may benefit from an alternative therapeutic approach2 • HCP survey3 indicates that physicians expect to prescribe teprotumumab to 75% of their TED patients by 2025, up from roughly 30% in March 2022 • 20%-35% of patients treated with teprotumumab may have residual symptoms warranting treatment4,5 • 25%-40% of patients treated with teprotumumab may experience a disease flare warranting additional TED treatment6 • 47-90% of total incidence potentially addressable For Investor Audiences Only Sources: 1. Lazarus JH et al. Best Practice & Research Clinical Endocrinology & Metabolism. v26 (2012) 273-279 . 2. HCP Qualitative Research, Immunovant, 2020 3. 2021 Cow en Equity Research, March 2022 - surveyed 25 clinicians w ho treat 3,000+ patients w ith TED annually 4. Horizon Therapeutics Investor Presentations. 5. Teprotumumab's US Prescribing Information. 6. Douglas R et al. American Academy of Ophthalmology, v129, No. 4,
Thyroid Eye Disease key take-aways 39 TED is a multi-faceted condition – presenting with a range of clinical manifestations and substantial impact on patient functioning and quality of life Data from batoclimab’s clinical program are encouraging for continued development in TED Batoclimabrepresents a potentially new and differentiated approach to treating TED 1 2 3 For Investor Audiences Only

Pete Salzmann, MD Chief Executive Officer Myasthenia gravis A multifaceted disease Pre-Record

41


43
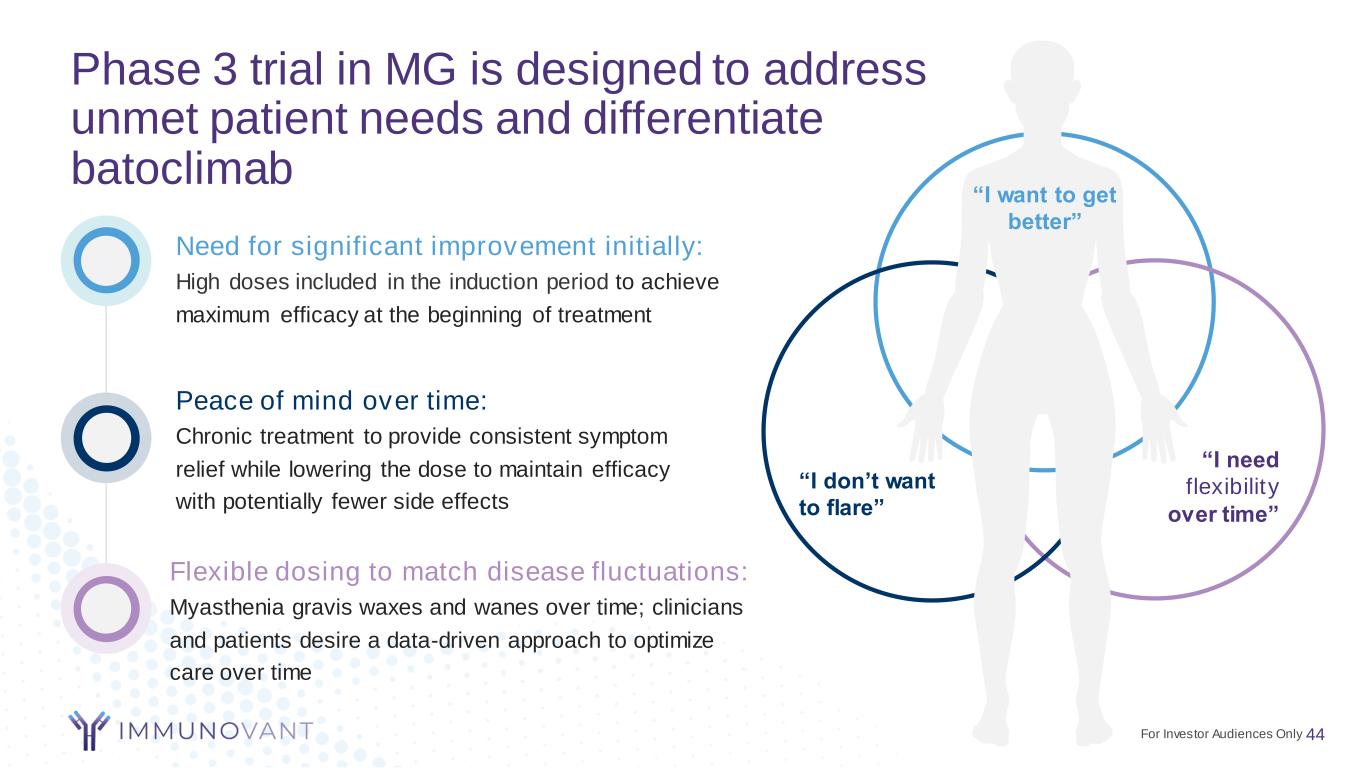
Phase 3 trial in MG is designed to address unmet patient needs and differentiate batoclimab 44 Flexible dosing to match disease fluctuations: Myasthenia gravis waxes and wanes over time; clinicians and patients desire a data-driven approach to optimize care over time Peace of mind over time: Chronic treatment to provide consistent symptom relief while lowering the dose to maintain efficacy with potentially fewer side effects Need for significant improvement initially: High doses included in the induction period to achieve maximum efficacy at the beginning of treatment “I need flexibility over time” “I don’t want to flare” “I want to get better” For Investor Audiences Only
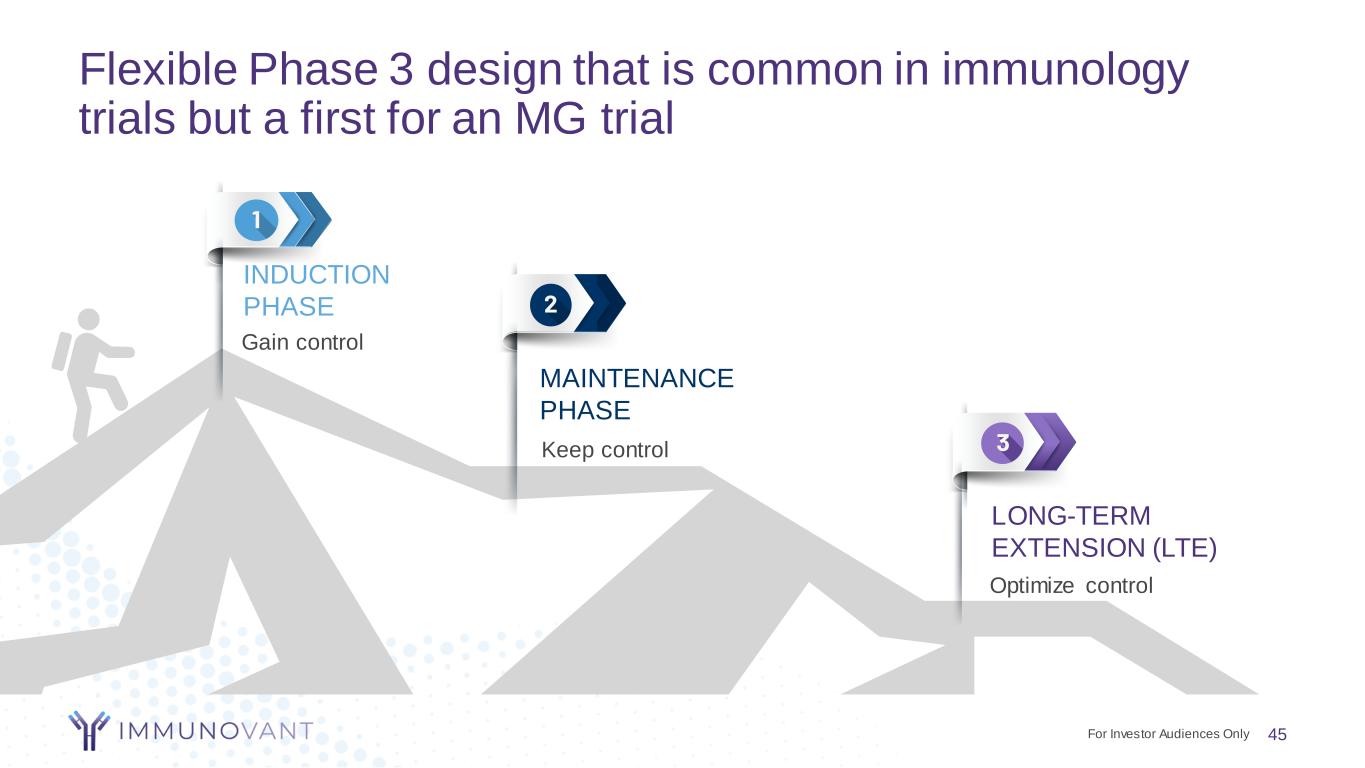
Flexible Phase 3 design that is common in immunology trials but a first for an MG trial 45 Gain control INDUCTION PHASE Keep control MAINTENANCE PHASE Optimize control LONG-TERM EXTENSION (LTE) For Investor Audiences Only
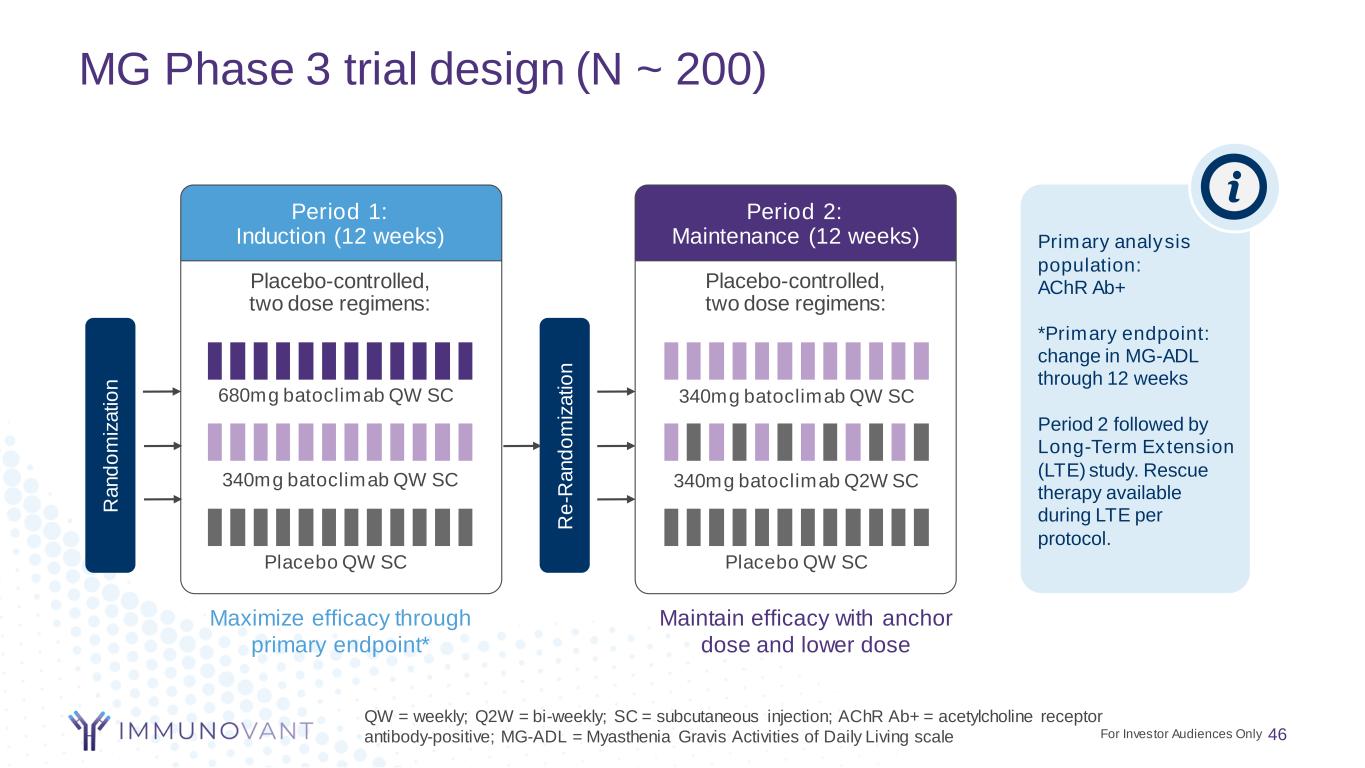
MG Phase 3 trial design (N ~ 200) 46 QW = weekly; Q2W = bi-weekly; SC = subcutaneous injection; AChR Ab+ = acetylcholine receptor antibody-positive; MG-ADL = Myasthenia Gravis Activities of Daily Living scale Placebo-controlled, two dose regimens: Period 1: Induction (12 weeks) Maximize efficacy through primary endpoint* Placebo-controlled, two dose regimens: Period 2: Maintenance (12 weeks) Maintain efficacy with anchor dose and lower dose Placebo QW SC Placebo QW SC 340mg batoclimab QW SC 680mg batoclimab QW SC 340mg batoclimab Q2W SC 340mg batoclimab QW SC Primary analysis population: AChR Ab+ *Primary endpoint: change in MG-ADL through 12 weeks Period 2 followed by Long-Term Extension (LTE) study. Rescue therapy available during LTE per protocol. R a n d o m iz a ti o n R e -R a n d o m iz a ti o n For Investor Audiences Only

Pete Salzmann, MD Chief Executive Officer Warm autoimmune hemolytic anemia Pre-Record

48

An Overview of Warm Autoimmune Haemolytic Anaemia (wAIHA) Dr David Tucker MD MRCP FRCPath Consultant Haematologist and Research Lead for Haematology Royal Cornwall Hospital NHS Trust, Cornwall, United Kingdom

Conflicts of Interest • Advisory Board – Roche, Abbvie, Novartis • Conference attendance: Amgen, Takeda Warm Auto-Immune Haemolytic Anaemia (wAIHA) 50
wAIHA represents a complex and fascinating challenge… • An unpredictable and potentially life-threatening auto-immune disease caused by antibody-mediated red cell destruction. • A rare disease with few large data-sets to guide management. • Corticosteroids are usually effective but with significant toxicity. • Patients often relapse or are unable to discontinue treatment in the long-term. • There is a lack of evidence for therapies beyond steroids and rituximab. • Enrolment into clinical trials is generally recommended to identify the optimal choice, sequence and combination of drugs Warm Auto-Immune Haemolytic Anaemia (wAIHA) 51For Investor Audiences Only
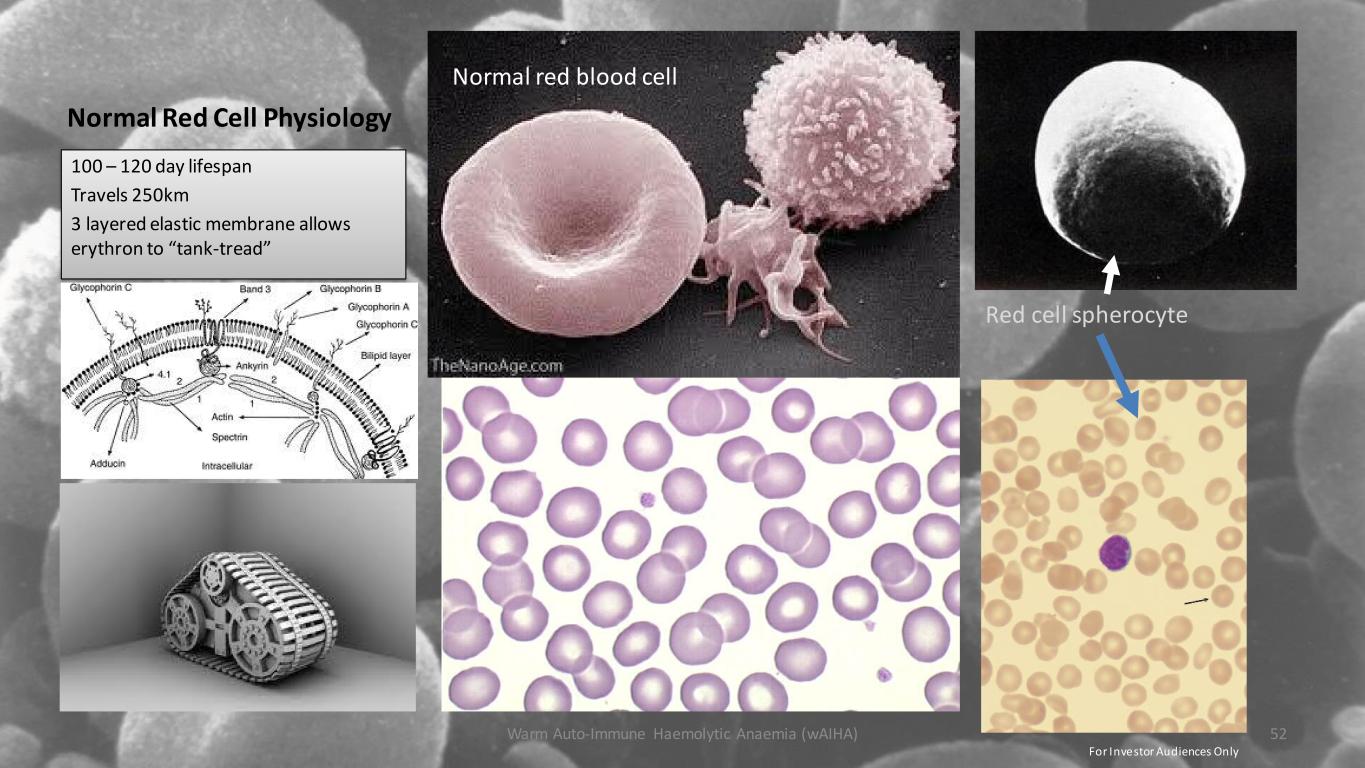
Normal Red Cell Physiology 100 – 120 day lifespan Travels 250km 3 layered elastic membrane allows erythron to “tank-tread” Warm Auto-Immune Haemolytic Anaemia (wAIHA) 52 Normal red blood cell Red cell spherocyte For Investor Audiences Only
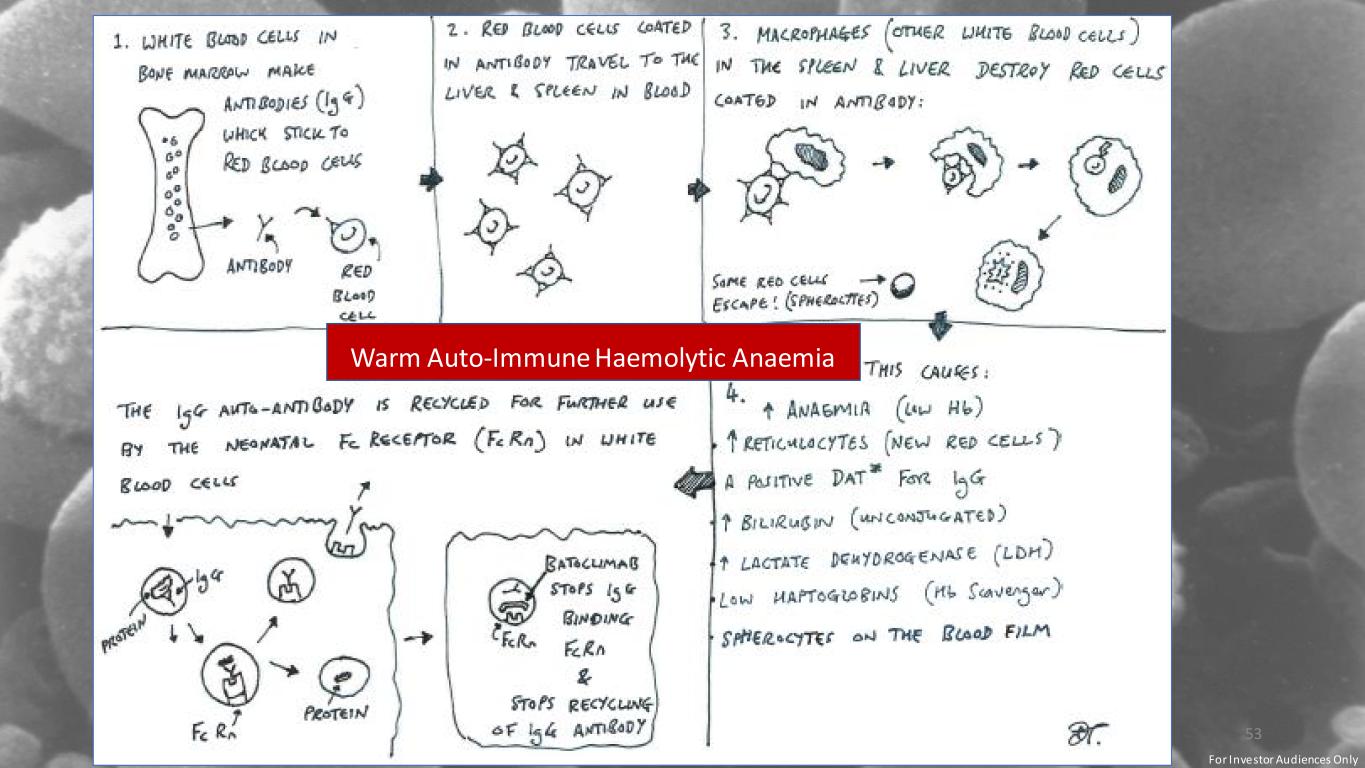
29/03/2022 Warm Auto-Immune Haemolytic Anaemia (wAIHA) 53 Warm Auto-Immune Haemolytic Anaemia For Investor Audiences Only
wAIHA – Who is Affected? Causes • Primary (Idiopathic) wAIHA • (40-60% of cases) (Hill et al. Roumier et al.) • Secondary wAIHA • (50% of cases) (Hill et al. Roumier et al.): • Auto-immune diseases e.g. SLE, ITP, Rheumatoid arthritis • Lymphoproliferative diseases e.g. CLL, Lymphoma (NHL and HL) • Infections (e.g. mycoplasma, EBV) Epidemiology • Annual incidence: • 1-3/100,000 (Eaton et al. 2007) • Prevalence: • ~ 0.17/1000 (Eaton et al. 2007) • Addressable patients (US): • approximately 40,000 (McCrae et al. 2021) Warm Auto-Immune Haemolytic Anaemia (wAIHA) 54For Investor Audiences Only
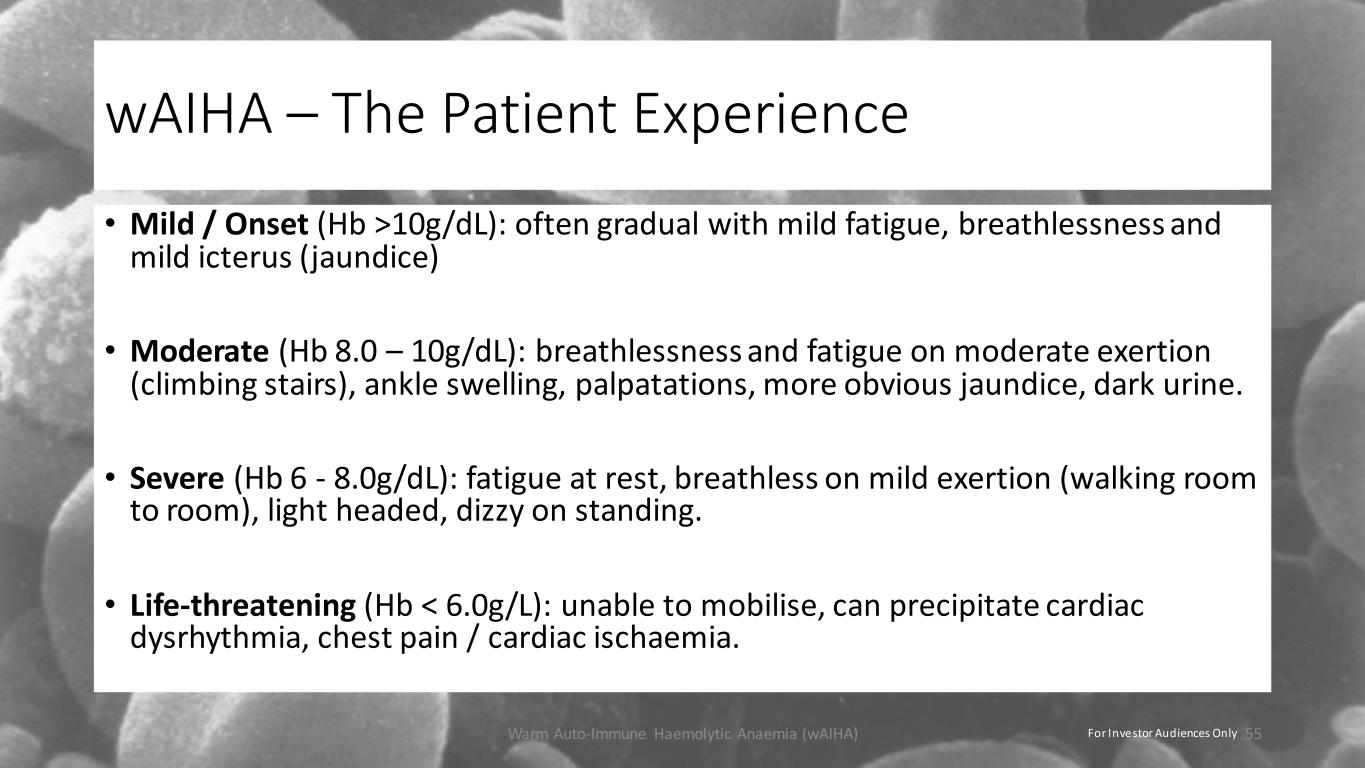
wAIHA – The Patient Experience • Mild / Onset (Hb >10g/dL): often gradual with mild fatigue, breathlessness and mild icterus (jaundice) • Moderate (Hb 8.0 – 10g/dL): breathlessness and fatigue on moderate exertion (climbing stairs), ankle swelling, palpatations, more obvious jaundice, dark urine. • Severe (Hb 6 - 8.0g/dL): fatigue at rest, breathless on mild exertion (walking room to room), light headed, dizzy on standing. • Life-threatening (Hb < 6.0g/L): unable to mobilise, can precipitate cardiac dysrhythmia, chest pain / cardiac ischaemia. Warm Auto-Immune Haemolytic Anaemia (wAIHA) 55For Investor Audiences Only

How do we manage wAIHA? • Because wAIHA is a rare disease there are few large data sets to guide management which is mainly empirical and based on expert opinion. • The cornerstone of management is immunosuppression with corticosteroids. • Historically, the treatment-related mortality rate is 8 to 15% • The major issue with treatment is that 60% of patients become steroid-dependent. (Roumier et al.) 29/03/2022 Warm Auto-Immune Haemolytic Anaemia (wAIHA) 56For Investor Audiences Only
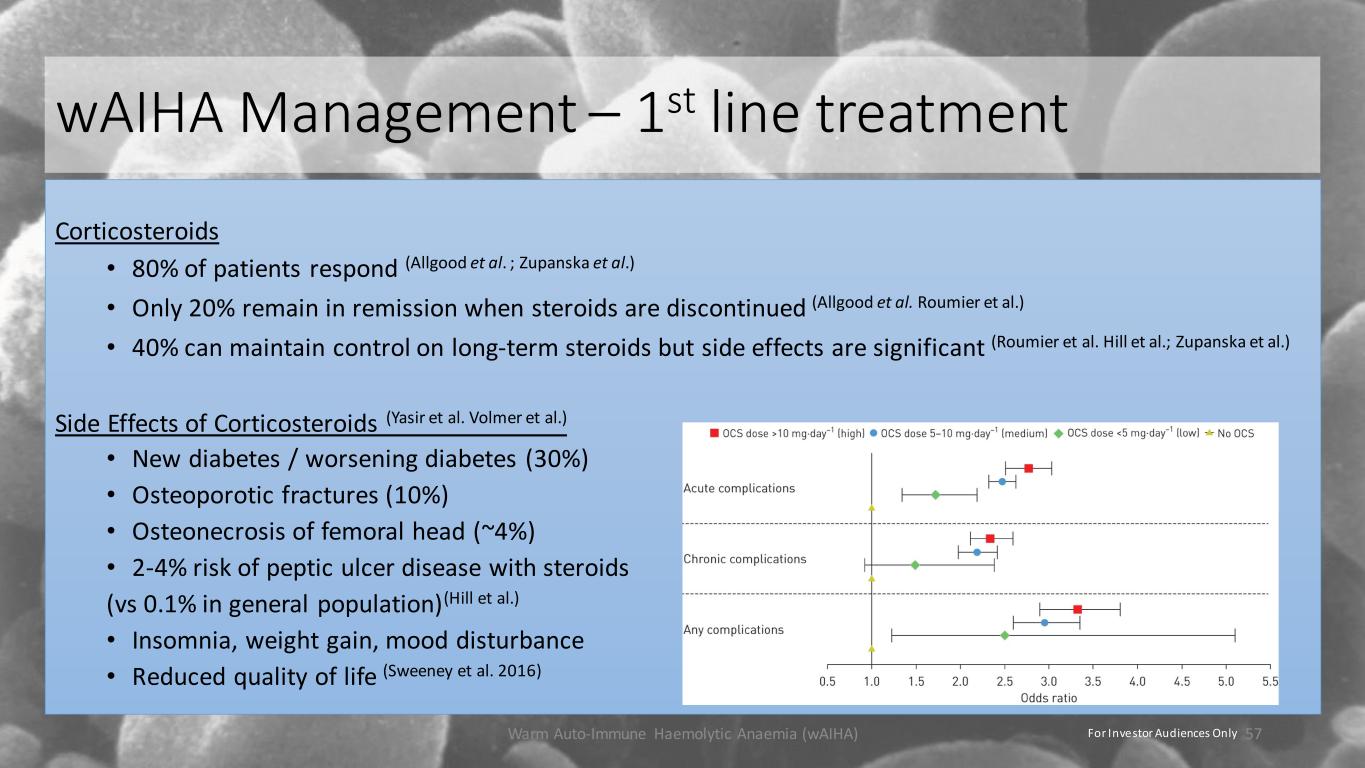
wAIHA Management – 1st line treatment Corticosteroids • 80% of patients respond (Allgood et al. ; Zupanska et al.) • Only 20% remain in remission when steroids are discontinued (Allgood et al. Roumier et al.) • 40% can maintain control on long-term steroids but side effects are significant (Roumier et al. Hill et al.; Zupanska et al.) Side Effects of Corticosteroids (Yasir et al. Volmer et al.) • New diabetes / worsening diabetes (30%) • Osteoporotic fractures (10%) • Osteonecrosis of femoral head (~4%) • 2-4% risk of peptic ulcer disease with steroids (vs 0.1% in general population)(Hill et al.) • Insomnia, weight gain, mood disturbance • Reduced quality of life (Sweeney et al. 2016) Warm Auto-Immune Haemolytic Anaemia (wAIHA) 57For Investor Audiences Only
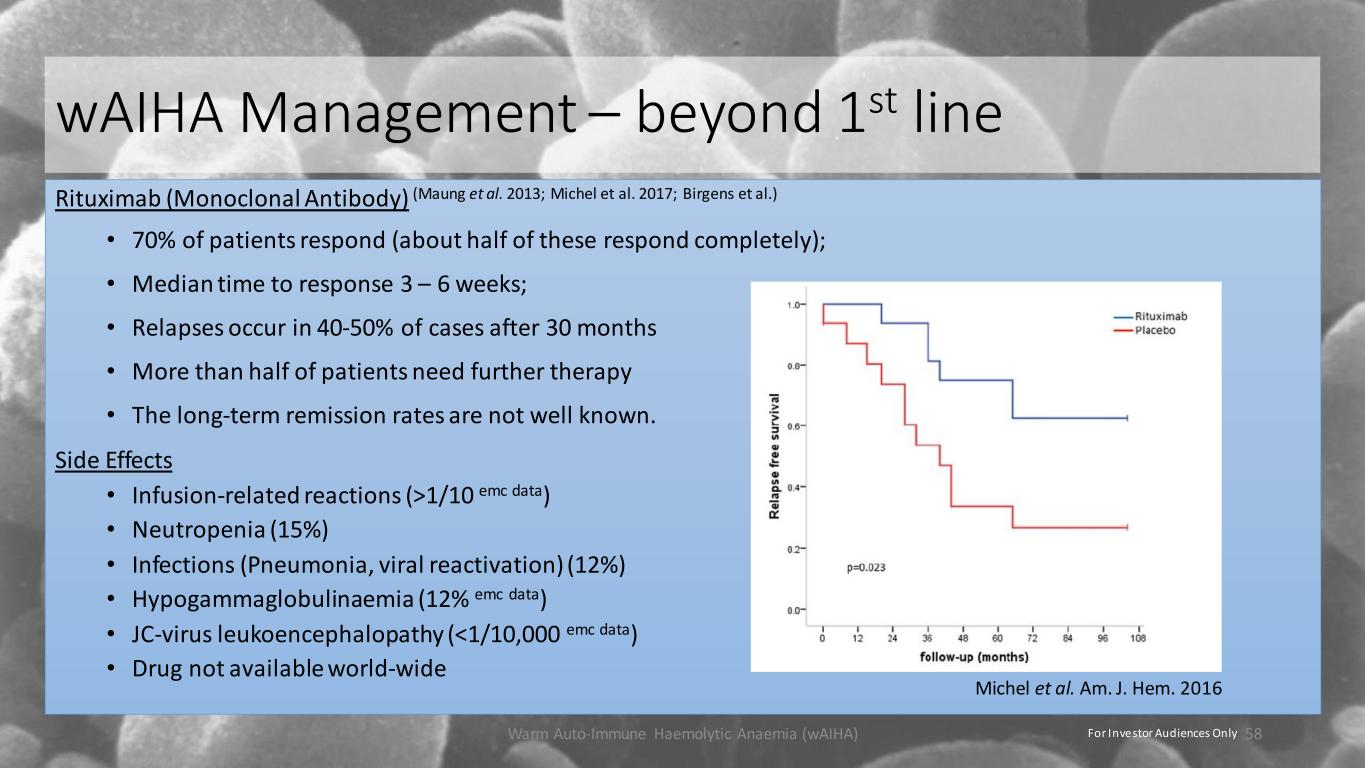
wAIHA Management – beyond 1st line Rituximab (Monoclonal Antibody) (Maung et al. 2013; Michel et al. 2017; Birgens et al.) • 70% of patients respond (about half of these respond completely); • Median time to response 3 – 6 weeks; • Relapses occur in 40-50% of cases after 30 months • More than half of patients need further therapy • The long-term remission rates are not well known. Side Effects • Infusion-related reactions (>1/10 emc data) • Neutropenia (15%) • Infections (Pneumonia, viral reactivation) (12%) • Hypogammaglobulinaemia (12% emc data) • JC-virus leukoencephalopathy (<1/10,000 emc data) • Drug not available world-wide Warm Auto-Immune Haemolytic Anaemia (wAIHA) 58 Michel et al. Am. J. Hem. 2016 For Investor Audiences Only
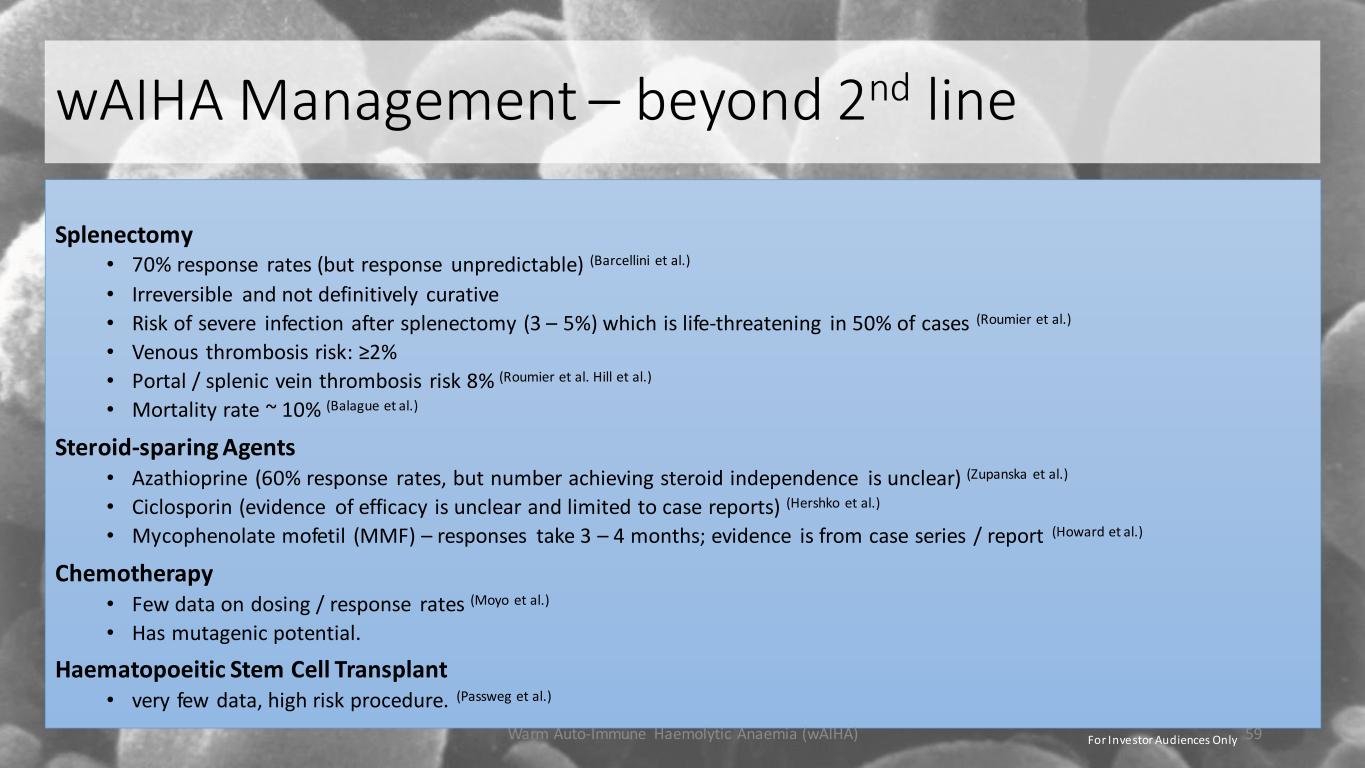
wAIHA Management – beyond 2nd line Splenectomy • 70% response rates (but response unpredictable) (Barcellini et al.) • Irreversible and not definitively curative • Risk of severe infection after splenectomy (3 – 5%) which is life-threatening in 50% of cases (Roumier et al.) • Venous thrombosis risk: ≥2% • Portal / splenic vein thrombosis risk 8% (Roumier et al. Hill et al.) • Mortality rate ~ 10% (Balague et al.) Steroid-sparing Agents • Azathioprine (60% response rates, but number achieving steroid independence is unclear) (Zupanska et al.) • Ciclosporin (evidence of efficacy is unclear and limited to case reports) (Hershko et al.) • Mycophenolate mofetil (MMF) – responses take 3 – 4 months; evidence is from case series / report (Howard et al.) Chemotherapy • Few data on dosing / response rates (Moyo et al.) • Has mutagenic potential. Haematopoeitic Stem Cell Transplant • very few data, high risk procedure. (Passweg et al.) Warm Auto-Immune Haemolytic Anaemia (wAIHA) 59For Investor Audiences Only

wAIHA – Evidence for a need for new therapeutic options In studies of patients with wAIHA over 3 – 4 years: • Less than half (47%) of patients remain in remission off treatment • 25% of patients remain on low dose steroids • 28% have ongoing disease and require higher dose steroids or other immunosuppressive drugs(Roumier et al. 2014) There are serious ongoing complications of uncontrolled wAIHA: • Risk of deep vein thrombosis (20%) (Hendrick 2003, Roumier et al.) • Risk of pulmonary embolus (8%) (Roumier et al.) • Excess mortality (8%) (Roumier et al.) Warm Auto-Immune Haemolytic Anaemia (wAIHA) 60For Investor Audiences Only

wAIHA In Summary • This is a rare disease with few large data-sets to guide management • There is an unmet need because a large proportion of patients remain on immunosuppressive therapy long-term • There is a significant side-effect burden for patients from long- term immunosuppression • There is a lack of evidence-based therapy beyond second line treatment. • Enrolment into clinical trials is generally recommended by treating physicians to identify the optimal choice, sequence and combination of drugs Warm Auto-Immune Haemolytic Anaemia (wAIHA) 61For Investor Audiences Only

References • Allgood JW, Chaplin J. Idiopathic acquired autoimmune hemolyticanemia. A review of forty-seven cases treated from 1955 through 1965., Am J Med, 1967, vol. 43 2(pg. 254-273) • Balague C, Targarona EM, Cerdan G, et al. Long-term outcome after laparoscopic splenectomy related to hematologic diagnosis., Surg Endoscop, 2004, vol. 18 8(pg. 1283-1287) • Barcellini W. Fattizzo B. Blood 2021How I treat warm autoimmune hemolytic anemia. • Birgens H, Frederiksen H, Hasselbalch HC, et al. A phase III randomized trial comparing glucocorticoid monotherapy versus glucocorticoid and rituximab in patients with autoimmune haemolytic anaemia. Br J Haematol. 2013;163(3):393-399. • Hershko C, Sonnenblick M, Ashkenazi J. Control of corticosteroid-resistant autoimmune haemolytic anaemia by cyclosporine., Br J Haematol, 1990, vol. 76 3(pg. 436-437) • Hendrick, A.M. (2003) Auto-immune haemolytic anaemia–a high-risk disorder for thromboembolism? Hematology, 8, 53–56. • Hill Q. Stams R, Massey E. et al. The diagnosis and management of primary autoimmune haemolytic anaemia. B. J. Haem. 2016 • Howard J, Hoffbrand AV, Prentice HG, Mehta A. Mycophenolate mofetil for the treatment of refractory auto-immune haemolytic anaemia and autoimmune thrombocytopenia purpura., Br J Haematol, 2002, vol. 117 3(pg. 712-715) • Michel et al. A randomized and double-blind controlled trial evaluatingthe safety and efficacy of rituximab for warm auto-immune hemolytic anemia in adults (the RAIHA study). Am. J. Hematology 2016. • Moyo VM, Smith D, Brodsky I, Crilley P, Jones RJ, Brodsky RA. High-dose cyclophosphamide for retractory autoimmnune hemolyticanemia., Blood, 2002, vol. 100 2(pg. 704-706) • McCrae K et al. 2000 Identification of a Warm Autoimmune Hemolytic Anemia (wAIHA) Population Using Predictive Analytics of a Known Clinically Profiled Cohort. Conference Proceedings ASH 2021 Session 101 • Passweg JR, Rabusin M, Musso M, et al. Haematopoietic stem cell transplantation for refractory autoimmune cytopenia., Br J Haematol, 2004, vol. 125 6(pg. 749-755) • Rizzoli, R., Adachi, J.D., Cooper, C., et al. (2012) Management of glucocorticoid-induced osteoporosis. Calcified Tissue International, 91, 225–243. • Roumier M. et al. Characteristics and outcome of warm autoimmune hemolyticanemia in adults: new insights based on a single centerexperience with 60 patients. Am. J. Hematology 2014 • Sweeney et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry Thorax 2016 • Yasir M; Amandeep Goyal; Sidharth Sonthalia.Corticosteroid Adverse Effects. • Zupanska B, Sylwestrowicz T, PawelskiS. The results of prolonged treatment of autoimmune haemolytic anaemia., Haematologia, 1981, vol. 14 4(pg. 425-433) Warm Auto-Immune Haemolytic Anaemia (wAIHA) 62For Investor Audiences Only

Thank you for listening Dr David Tucker Warm Auto-Immune Haemolytic Anaemia (wAIHA)

Bill Macias, MD Chief Medical Officer Cholesterol management Pre-Record

Relationship between CVD and Hypercholesterolemia • The causes of cardiovascular disease (CVD) are multifactorial – Modifiable risk factors: lifestyle (especially an unhealthy diet) tobacco use and sedentary habits, high blood pressure, diabetes and dyslipidemias – Nonmodifiable risk factors: age, gender • Control of lipid levels is one of the most effective strategies for CVD prevention • Epidemiologic data have demonstrated the crucial role of dyslipidemia, especially hypercholesterolemia, in the development of CVD • It is well understood that accumulation of cholesterol-rich low-density lipoprotein (LDL-C) over time leads to formation of lipid-laden foam cells and proliferation of atherosclerotic lesions, increasing the risk of CVD Agabiti Rosei E, Salvetti M. High Blood Press Cardiovasc Prev. 2016;23(3):217-230. 65For Investor Audiences Only
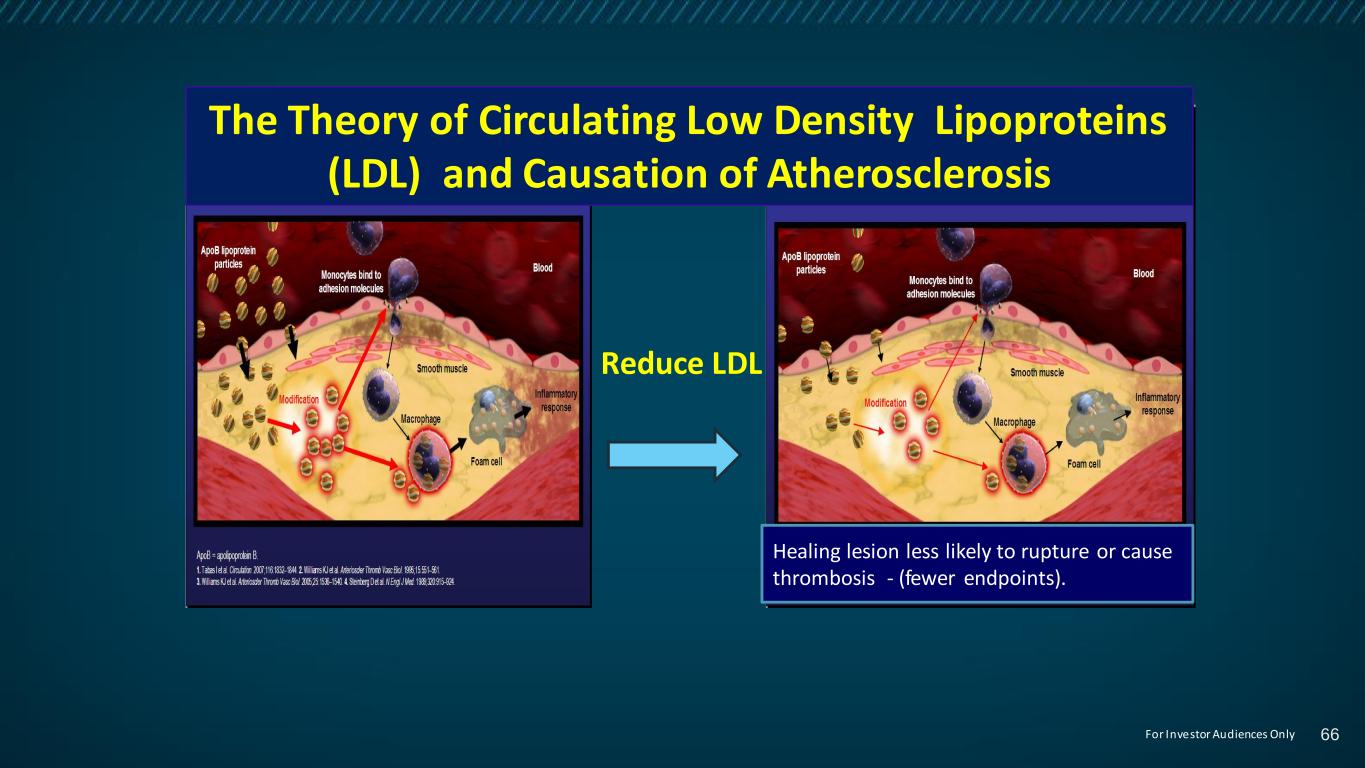
14 Reduce LDL 20 The Theory of Circulating Low Density Lipoproteins (LDL) and Causation of Atherosclerosis Healing lesion less likely to rupture or cause thrombosis - (fewer endpoints). 66For Investor Audiences Only
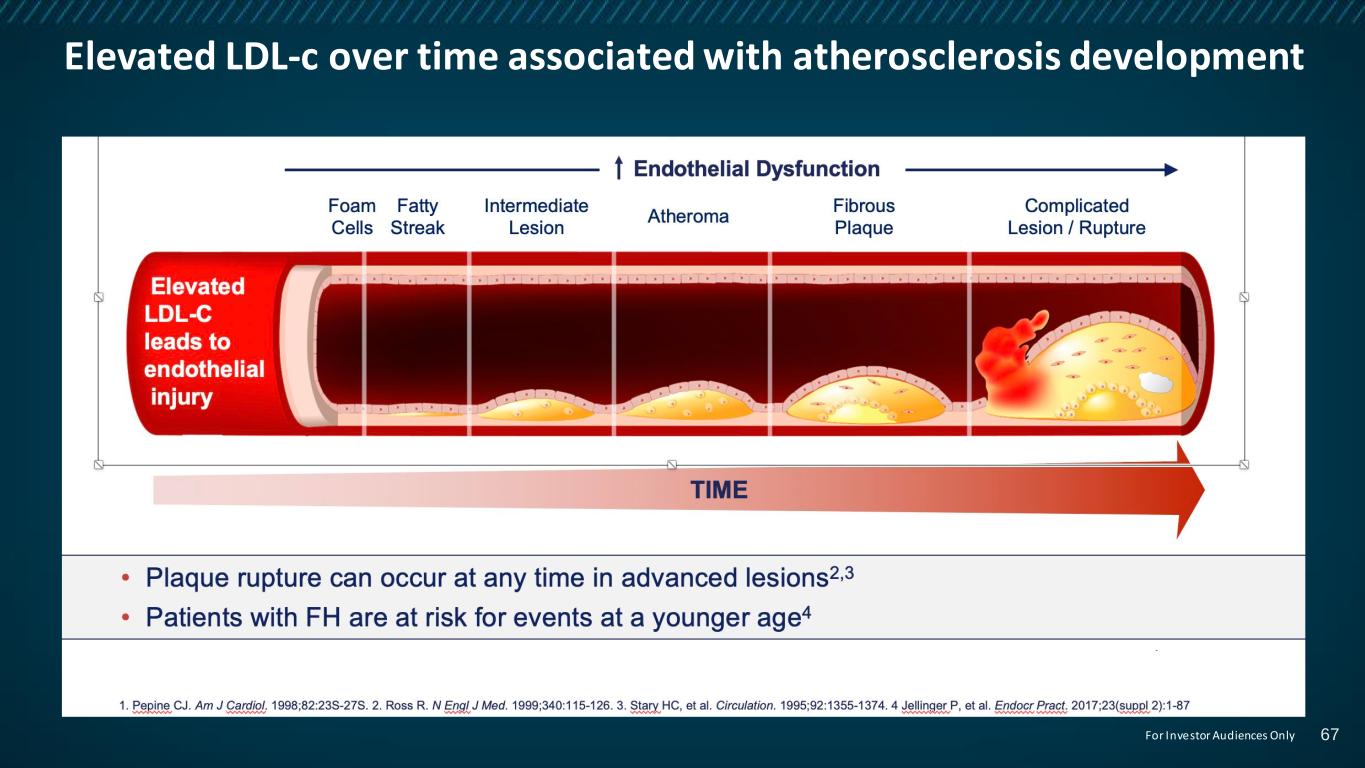
Elevated LDL-c over time associated with atherosclerosis development 67For Investor Audiences Only
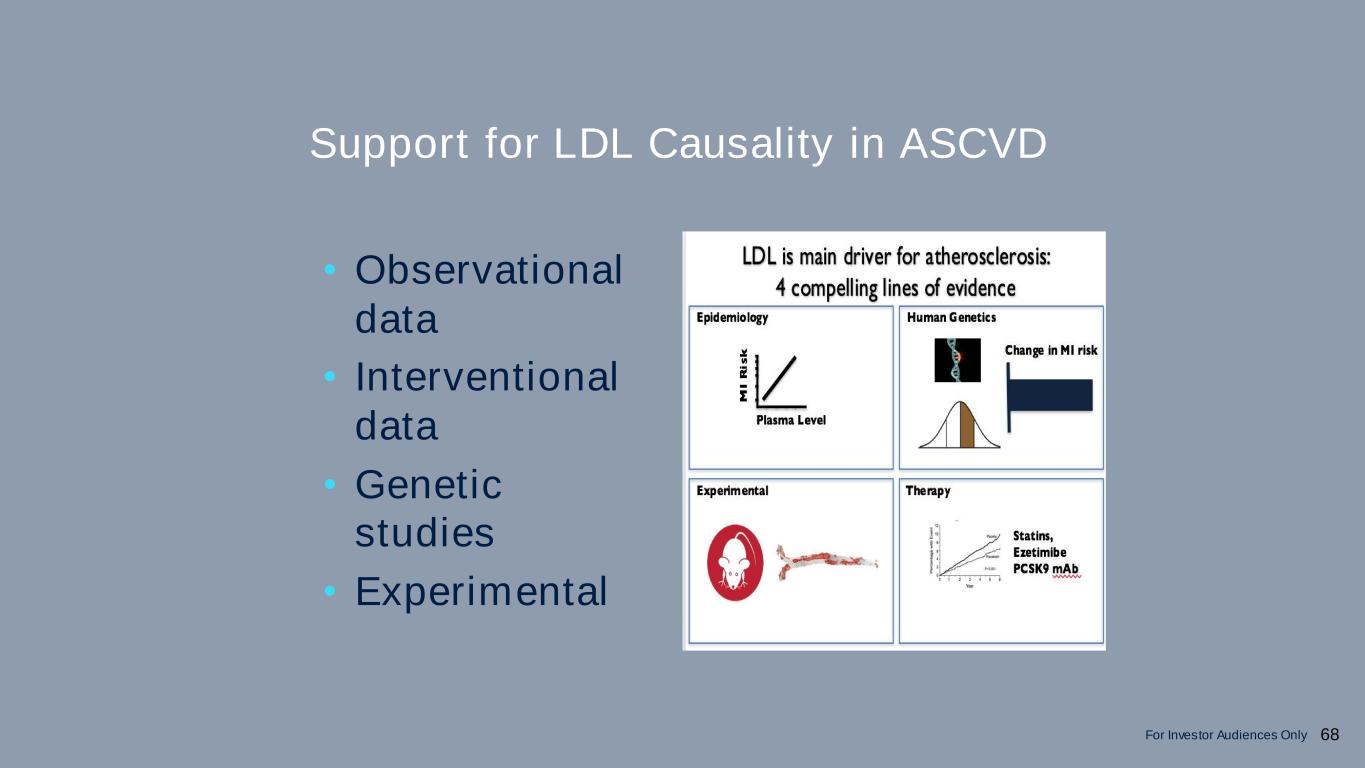
Support for LDL Causality in ASCVD • Observational data • Interventional data • Genetic studies • Experimental 68For Investor Audiences Only
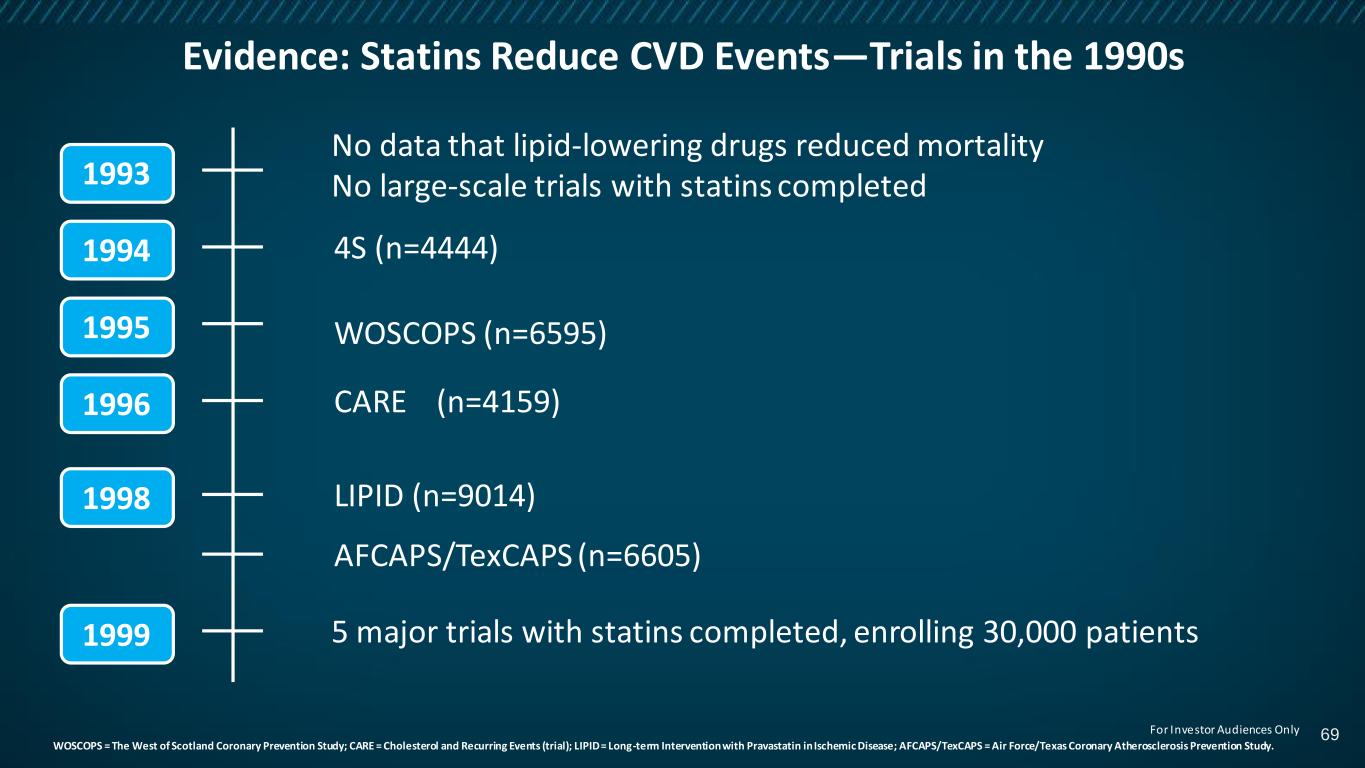
Evidence: Statins Reduce CVD Events—Trials in the 1990s WOSCOPS = The West of Scotland Coronary Prevention Study; CARE = Cholesterol and Recurring Events (trial); LIPID = Long-term Intervention with Pravastatin in Ischemic Disease; AFCAPS/TexCAPS = Air Force/Texas Coronary Atherosclerosis Prevention Study. 1999 1996 1995 1994 1993 1998 No data that lipid-lowering drugs reduced mortality No large-scale trials with statins completed 5 major trials with statins completed, enrolling 30,000 patients 4S (n=4444) WOSCOPS (n=6595) CARE (n=4159) LIPID (n=9014) AFCAPS/TexCAPS (n=6605) 69For Investor Audiences Only
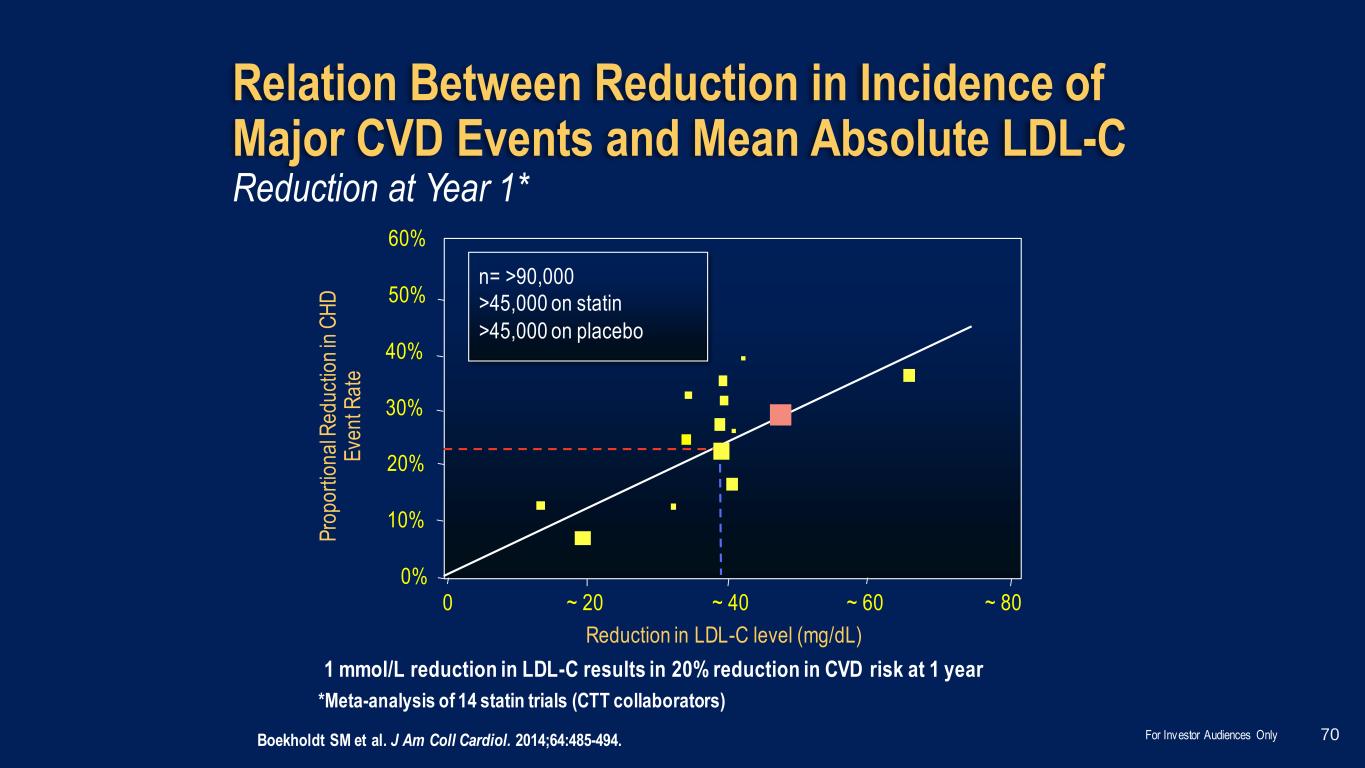
Boekholdt SM et al. J Am Coll Cardiol. 2014;64:485-494. Relation Between Reduction in Incidence of Major CVD Events and Mean Absolute LDL-C *Meta-analysis of 14 statin trials (CTT collaborators) Reduction in LDL-C level (mg/dL) P ro p o rt io n a l R e d u ct io n in C H D E ve n t R a te n= >90,000 >45,000 on statin >45,000 on placebo 1 mmol/L reduction in LDL-C results in 20% reduction in CVD risk at 1 year 0% 10% 20% 30% 40% 50% 60% 0 ~ 20 ~ 40 ~ 60 ~ 80 Reduction at Year 1* 70For Investor Audiences Only
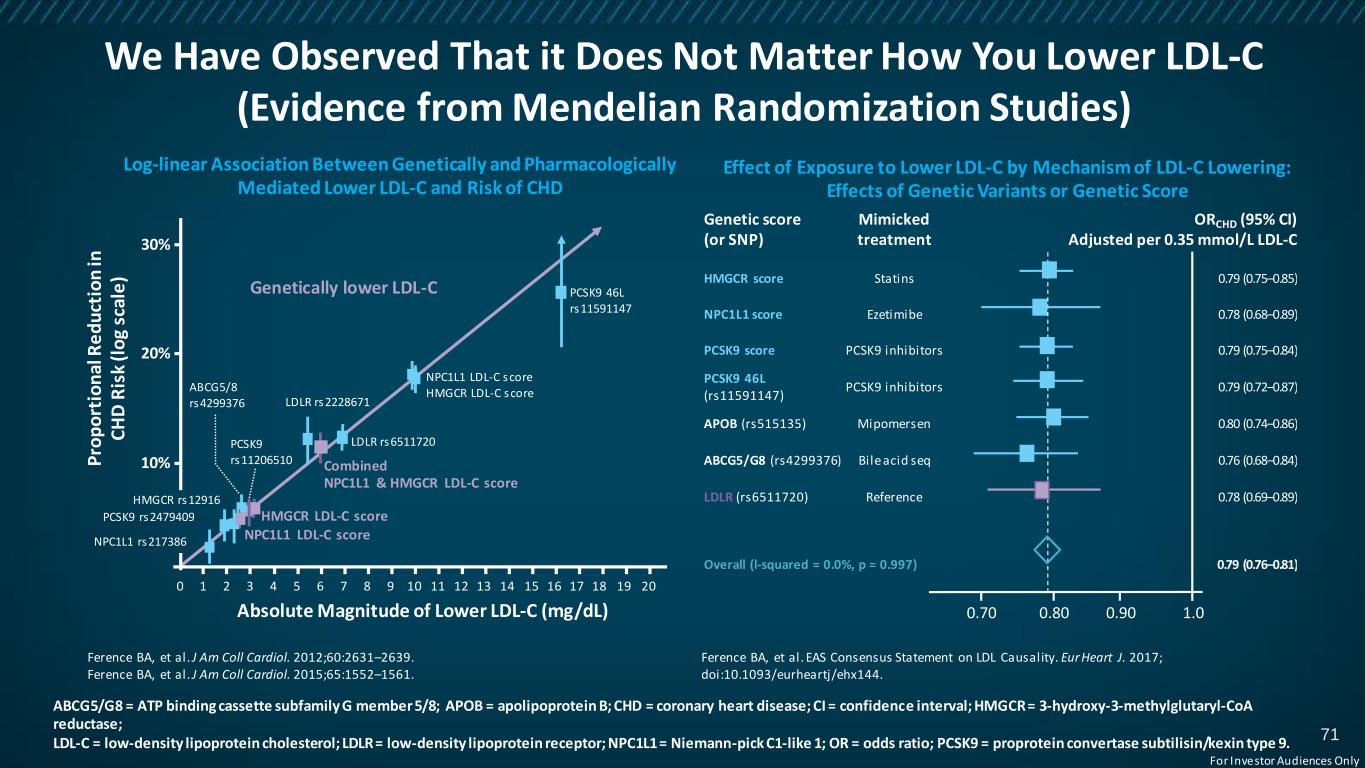
We Have Observed That it Does Not Matter How You Lower LDL-C (Evidence from Mendelian Randomization Studies) ABCG5/G8 = ATP binding cassette subfamily G member 5/8; APOB = apolipoprotein B; CHD = coronary heart disease; CI = confidence interval; HMGCR = 3-hydroxy-3-methylglutaryl-CoA reductase; LDL-C = low-density lipoprotein cholesterol; LDLR = low-density lipoprotein receptor; NPC1L1 = Niemann-pick C1-like 1; OR = odds ratio; PCSK9 = proprotein convertase subtilisin/kexin type 9. Log-linear Association Between Genetically and Pharmacologically Mediated Lower LDL-C and Risk of CHD Effect of Exposure to Lower LDL-C by Mechanism of LDL-C Lowering: Effects of Genetic Variants or Genetic Score Ference BA, et al. J Am Coll Cardiol. 2012;60:2631–2639. Ference BA, et al. J Am Coll Cardiol. 2015;65:1552–1561. Ference BA, et al. EAS Consensus Statement on LDL Causality. Eur Heart J. 2017; doi:10.1093/eurheartj/ehx144. 0 Absolute Magnitude of Lower LDL-C (mg/dL) P ro p o rt io n al R e d u ct io n in C H D R is k (l o g sc al e ) 30% 20% 10% 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 PCSK9 46L rs11591147 LDLR rs6511720 NPC1L1 LDL-C score HMGCR LDL-C score LDLR rs2228671 Genetically lower LDL-C Combined NPC1L1 & HMGCR LDL-C score HMGCR LDL-C score NPC1L1 LDL-C scoreNPC1L1 rs217386 PCSK9 rs2479409 PCSK9 rs11206510 ABCG5/8 rs4299376 HMGCR rs12916 Genetic score (or SNP) Mimicked treatment ORCHD (95% CI) Adjusted per 0.35 mmol/L LDL-C HMGCR score Statins 0.79 (0.75–0.85) NPC1L1 score Ezetimibe 0.78 (0.68–0.89) PCSK9 score PCSK9 inhibitors 0.79 (0.75–0.84) PCSK9 46L (rs11591147) PCSK9 inhibitors 0.79 (0.72–0.87) APOB (rs515135) Mipomersen 0.80 (0.74–0.86) ABCG5/G8 (rs4299376) Bile acid seq 0.76 (0.68–0.84) LDLR (rs6511720) Reference 0.78 (0.69–0.89) Overall (l-squared = 0.0%, p = 0.997) 0.79 (0.76–0.81) 0.70 0.80 0.90 1.0 71 For Investor Audiences Only
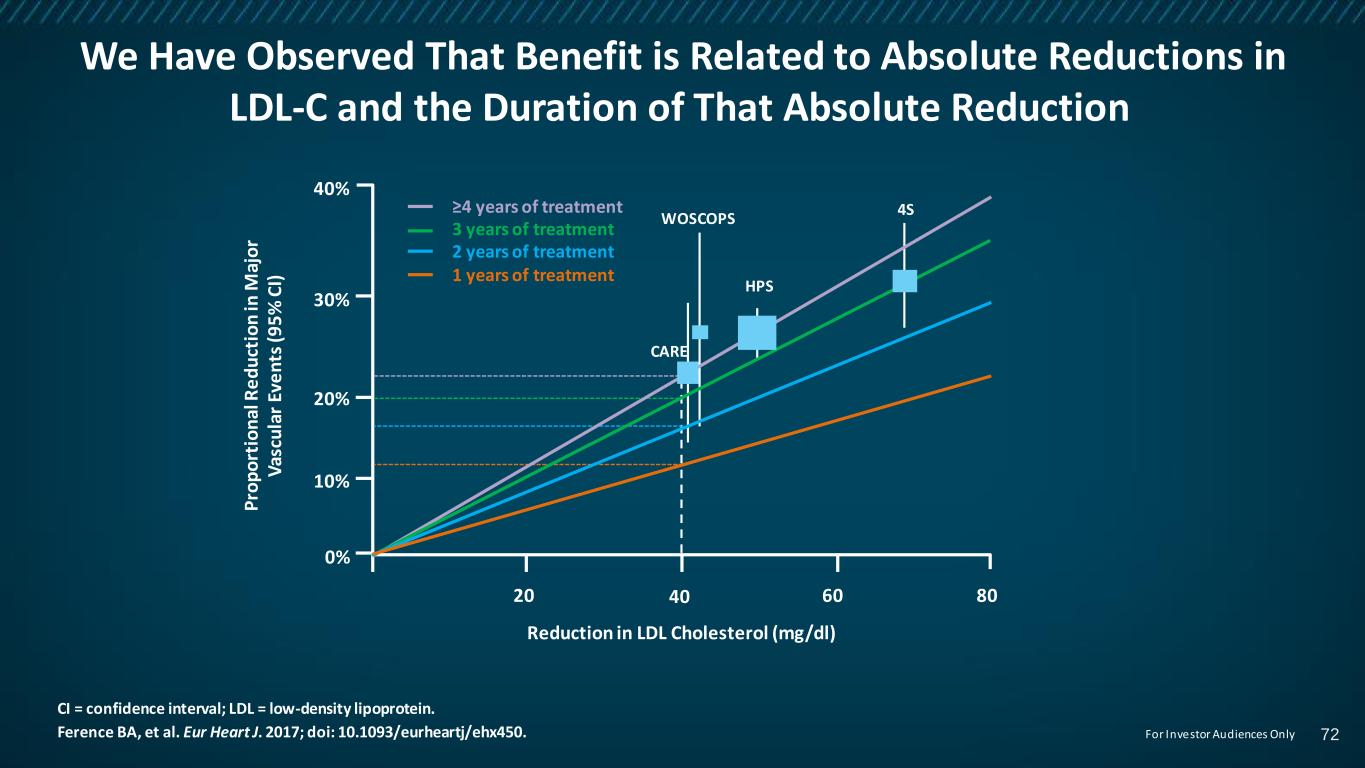
We Have Observed That Benefit is Related to Absolute Reductions in LDL-C and the Duration of That Absolute Reduction CI = confidence interval; LDL = low-density lipoprotein. Ference BA, et al. Eur Heart J. 2017; doi: 10.1093/eurheartj/ehx450. 4S HPS WOSCOPS 80604020 40% 20% 30% 10% 0% CARE P ro p o rt io n al R e d u ct io n in M aj o r V as cu la r Ev e n ts (9 5 % C I) Reduction in LDL Cholesterol (mg/dl) ≥4 years of treatment 3 years of treatment 2 years of treatment 1 years of treatment 72For Investor Audiences Only
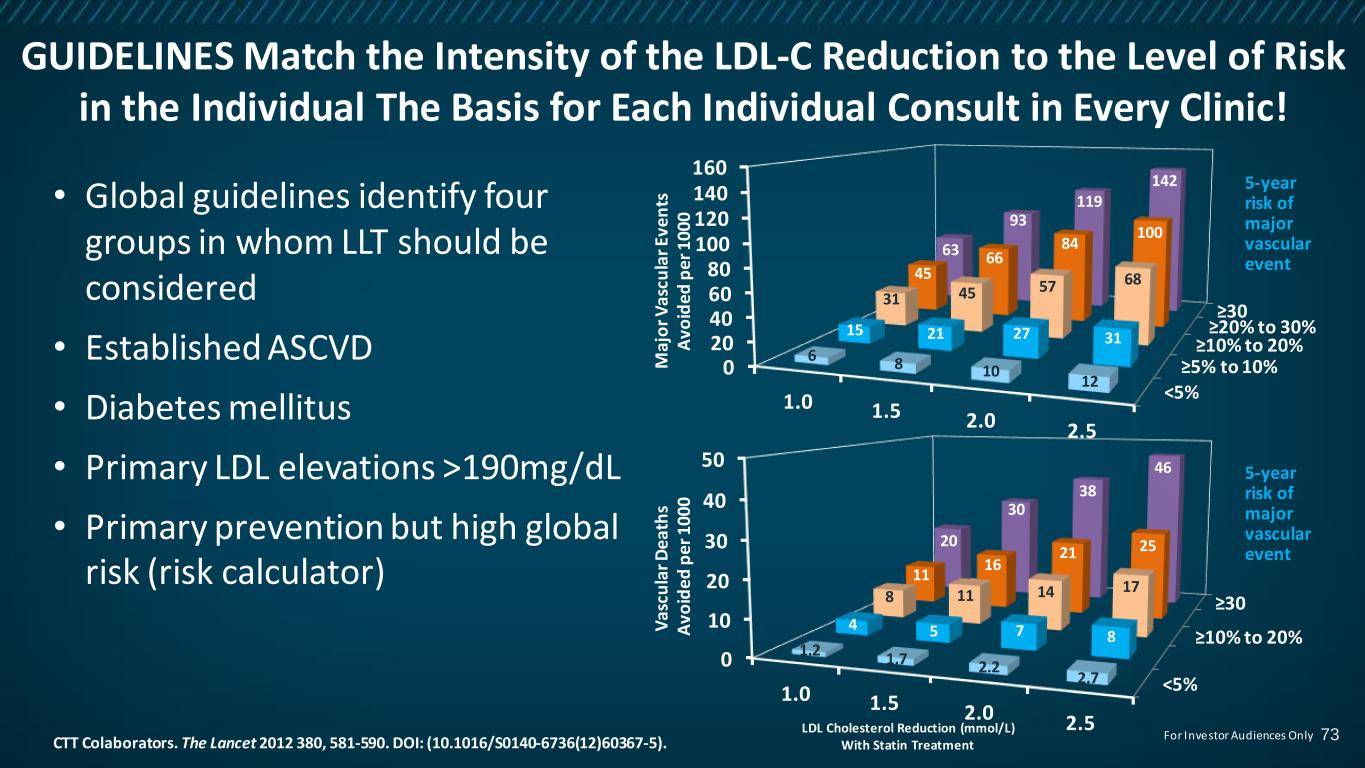
<5% ≥10% to 20% ≥30 0 10 20 30 40 50 1.0 1.5 2.0 2.5 1.2 1.7 2.2 2.7 4 5 7 8 8 11 14 17 11 16 21 2520 30 38 46 GUIDELINES Match the Intensity of the LDL-C Reduction to the Level of Risk in the Individual The Basis for Each Individual Consult in Every Clinic! • Global guidelines identify four groups in whom LLT should be considered • Established ASCVD • Diabetes mellitus • Primary LDL elevations >190mg/dL • Primary prevention but high global risk (risk calculator) CTT Colaborators. The Lancet 2012 380, 581-590. DOI: (10.1016/S0140-6736(12)60367-5). LDL Cholesterol Reduction (mmol/L) With Statin Treatment V as cu la r D ea th s A vo id ed p er 1 00 0 <5% ≥5% to 10% ≥10% to 20% ≥20% to 30% ≥30 0 20 40 60 80 100 120 140 160 1.0 1.5 2.0 2.5 6 8 10 12 15 21 27 31 31 45 57 6845 66 84 100 63 93 119 142 M aj o r V as cu la r E ve n ts A vo id ed p er 1 00 0 5-year risk of major vascular event 5-year risk of major vascular event 73For Investor Audiences Only
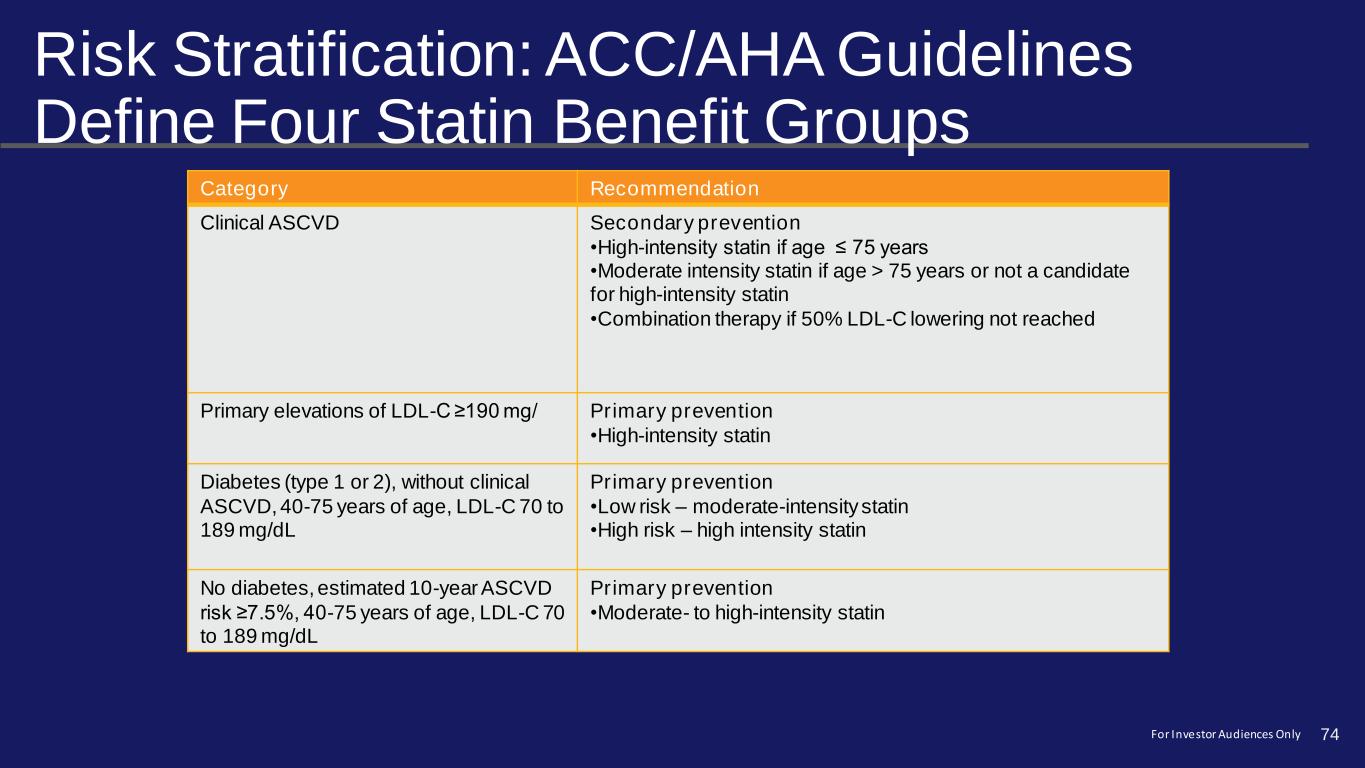
Risk Stratification: ACC/AHA Guidelines Define Four Statin Benefit Groups Category Recommendation Clinical ASCVD Secondary prevention •High-intensity statin if age ≤ 75 years •Moderate intensity statin if age > 75 years or not a candidate for high-intensity statin •Combination therapy if 50% LDL-C lowering not reached Primary elevations of LDL-C ≥190 mg/ Primary prevention •High-intensity statin Diabetes (type 1 or 2), without clinical ASCVD, 40-75 years of age, LDL-C 70 to 189 mg/dL Primary prevention •Low risk – moderate-intensity statin •High risk – high intensity statin No diabetes, estimated 10-year ASCVD risk ≥7.5%, 40-75 years of age, LDL-C 70 to 189 mg/dL Primary prevention •Moderate- to high-intensity statin 74For Investor Audiences Only
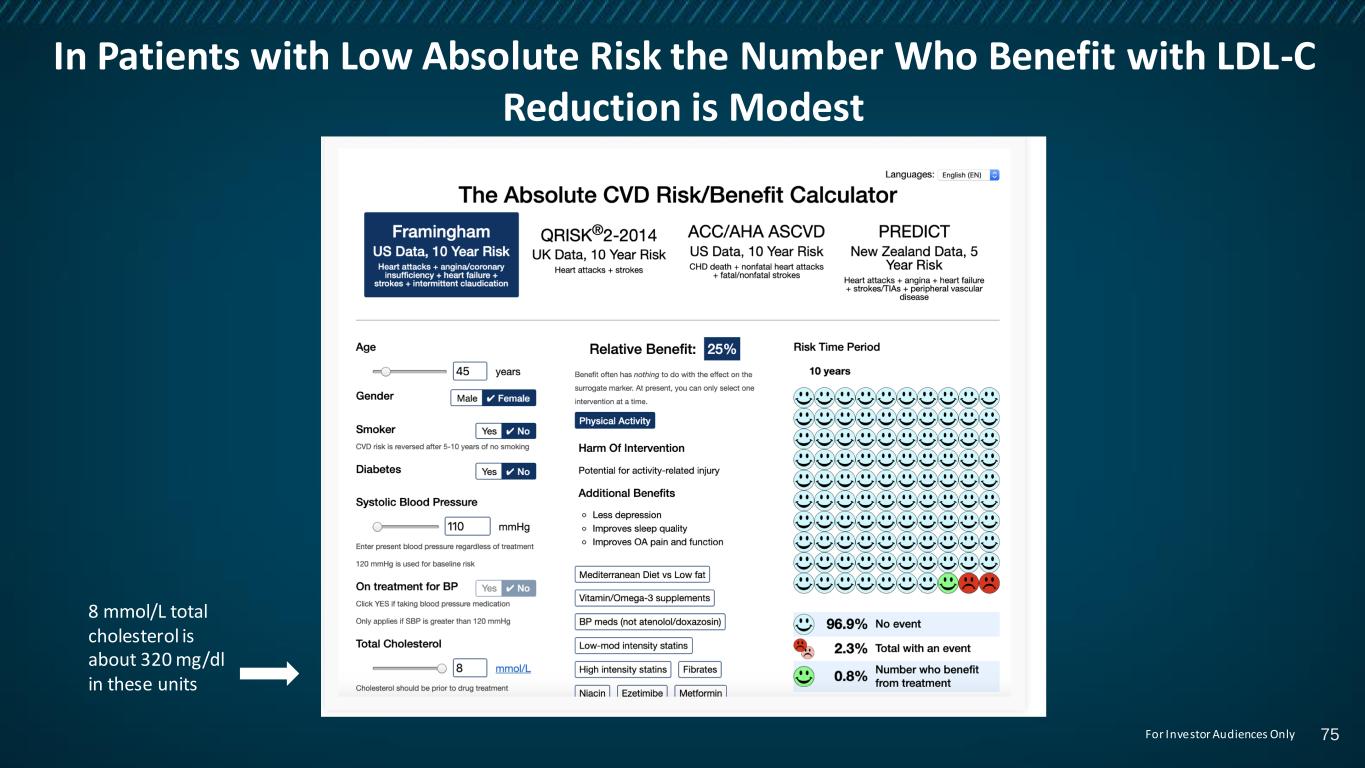
In Patients with Low Absolute Risk the Number Who Benefit with LDL-C Reduction is Modest 8 mmol/L total cholesterol is about 320 mg/dl in these units 75For Investor Audiences Only
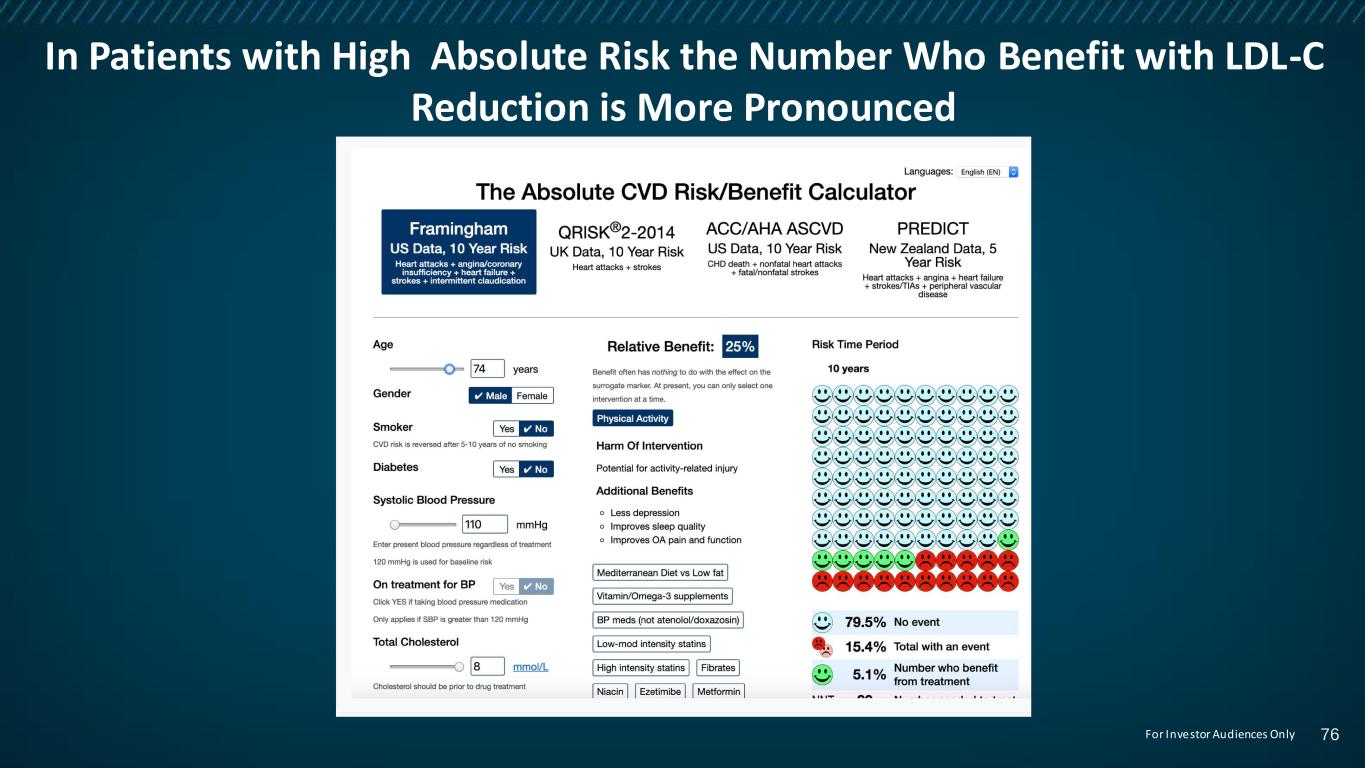
In Patients with High Absolute Risk the Number Who Benefit with LDL-C Reduction is More Pronounced 76For Investor Audiences Only
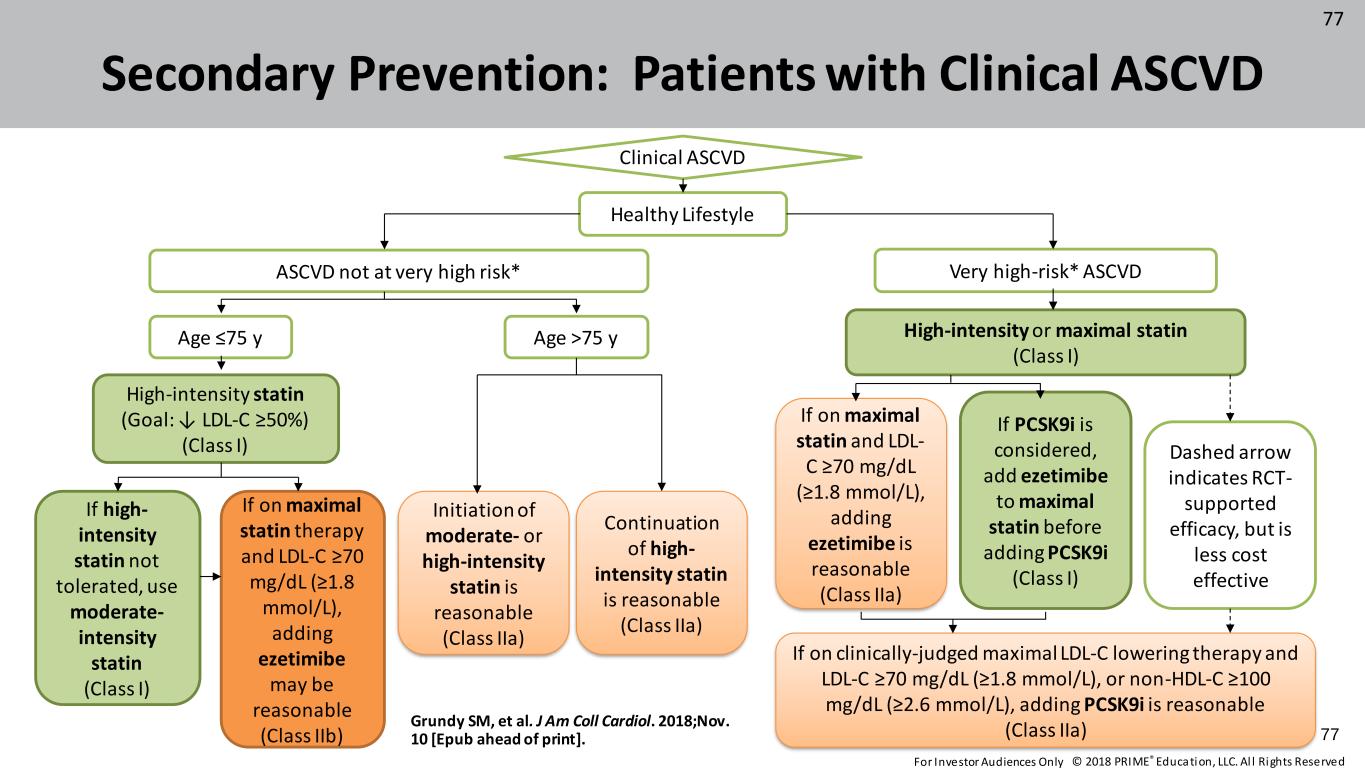
© 2018 PRIME® Education, LLC. Al l Rights Reserved. 77 Secondary Prevention: Patients with Clinical ASCVD Clinical ASCVD Healthy Lifestyle ASCVD not at very high risk* Very high-risk* ASCVD Age ≤75 y Age >75 y High-intensity or maximal statin (Class I) High-intensity statin (Goal: ↓ LDL-C ≥50%) (Class I) If high- intensity statin not tolerated, use moderate- intensity statin (Class I) If on maximal statin therapy and LDL-C ≥70 mg/dL (≥1.8 mmol/L), adding ezetimibe may be reasonable (Class IIb) Initiation of moderate- or high-intensity statin is reasonable (Class IIa) Continuation of high- intensity statin is reasonable (Class IIa) If on maximal statin and LDL- C ≥70 mg/dL (≥1.8 mmol/L), adding ezetimibe is reasonable (Class IIa) If PCSK9i is considered, add ezetimibe to maximal statin before adding PCSK9i (Class I) Dashed arrow indicates RCT- supported efficacy, but is less cost effective If on clinically-judged maximal LDL-C lowering therapy and LDL-C ≥70 mg/dL (≥1.8 mmol/L), or non-HDL-C ≥100 mg/dL (≥2.6 mmol/L), adding PCSK9i is reasonable (Class IIa)Grundy SM, et al. J Am Coll Cardiol. 2018;Nov. 10 [Epub ahead of print]. 77 For Investor Audiences Only
Conclusions • LDL-C is causal for atherosclerotic cardiovascular disease • The risk is determined by the absolute levels of LDL-C and the duration of the elevation measured in years (i.e cholesterol-years) • National guidelines have been developed to match the intensity of therapy to the absolute risk of the patient • Statins due to LDL-C lowering efficacy, safety, proven CV benefits and cost are the primary therapy for the treatment of elevated LDL-C • In medical practice, there are a number of therapies that increase LDL-C such as SGLT2 inhibitors and beta blockers that have proven CV benefits or anti-cytokine therapy such as IL-6 inhibitors in which potential elevations are managed with statin therapy • In general, LDL-C elevation with a therapy (or a lifestyle intervention, for example the keto diet) should be judged based on the absolute risk vs benefits 78For Investor Audiences Only

Cholesterol management Healthy Volunteer study preliminary data
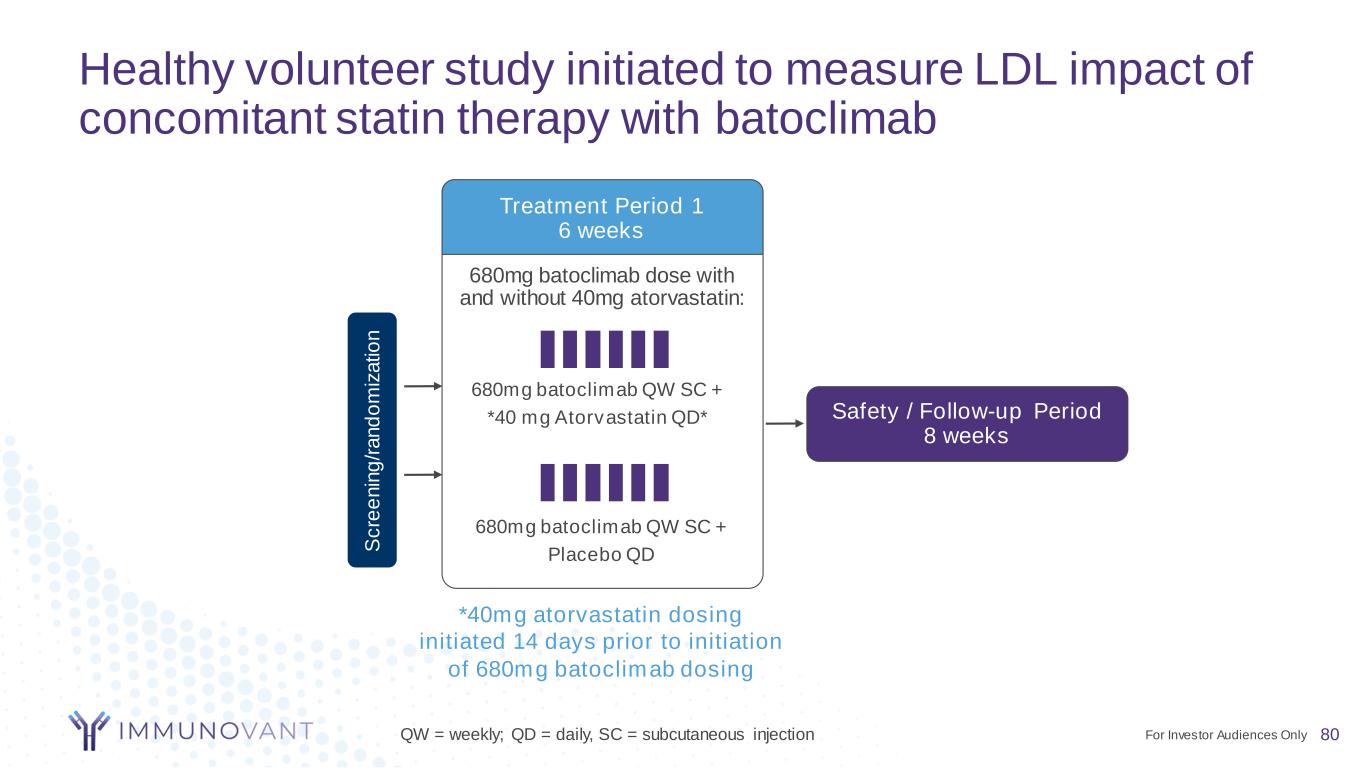
Healthy volunteer study initiated to measure LDL impact of concomitant statin therapy with batoclimab 680mg batoclimab dose with and without 40mg atorvastatin: Treatment Period 1 6 weeks *40mg atorvastatin dosing initiated 14 days prior to initiation of 680mg batoclimab dosing 680mg batoclimab QW SC + Placebo QD 680mg batoclimab QW SC + *40 mg Atorvastatin QD* S c re e n in g /r a n d o m iz a ti o n Safety / Follow-up Period 8 weeks QW = weekly; QD = daily, SC = subcutaneous injection 80For Investor Audiences Only
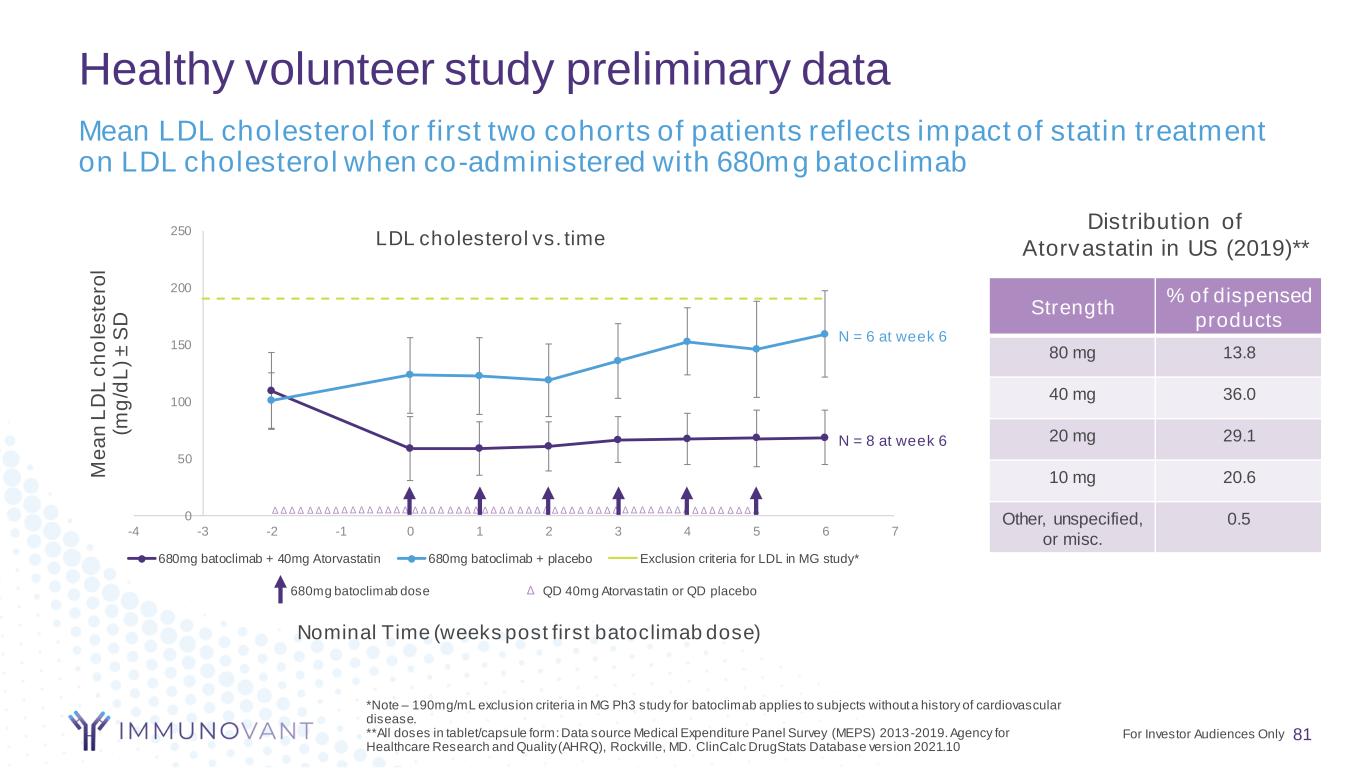
0 50 100 150 200 250 -4 -3 -2 -1 0 1 2 3 4 5 6 7 M e a n L D L c h o le s te ro l (m g /d L ) ± S D LDL cholesterol vs. time 680mg batoclimab + 40mg Atorvastatin 680mg batoclimab + placebo Exclusion criteria for LDL in MG study* Healthy volunteer study preliminary data 81 Mean LDL cholesterol for first two cohorts of patients reflects impact of statin treatment on LDL cholesterol when co-administered with 680mg batoclimab Nominal Time (weeks post first batoclimabdose) *Note – 190mg/mL exclusion criteria in MG Ph3 study for batoclimab applies to subjects without a history of cardiovascular disease. **All doses in tablet/capsule form: Data source Medical Expenditure Panel Survey (MEPS) 2013-2019. Agency for Healthcare Research and Quality (AHRQ), Rockville, MD. ClinCalc DrugStats Database version 2021.10 Strength % of dispensed products 80 mg 13.8 40 mg 36.0 20 mg 29.1 10 mg 20.6 Other, unspecified, or misc. 0.5 Distribution of Atorvastatin in US (2019)** 680mg batoclimab dose QD 40mg Atorvastatin or QD placebo Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ N = 8 at week 6 N = 6 at week 6 For Investor Audiences Only
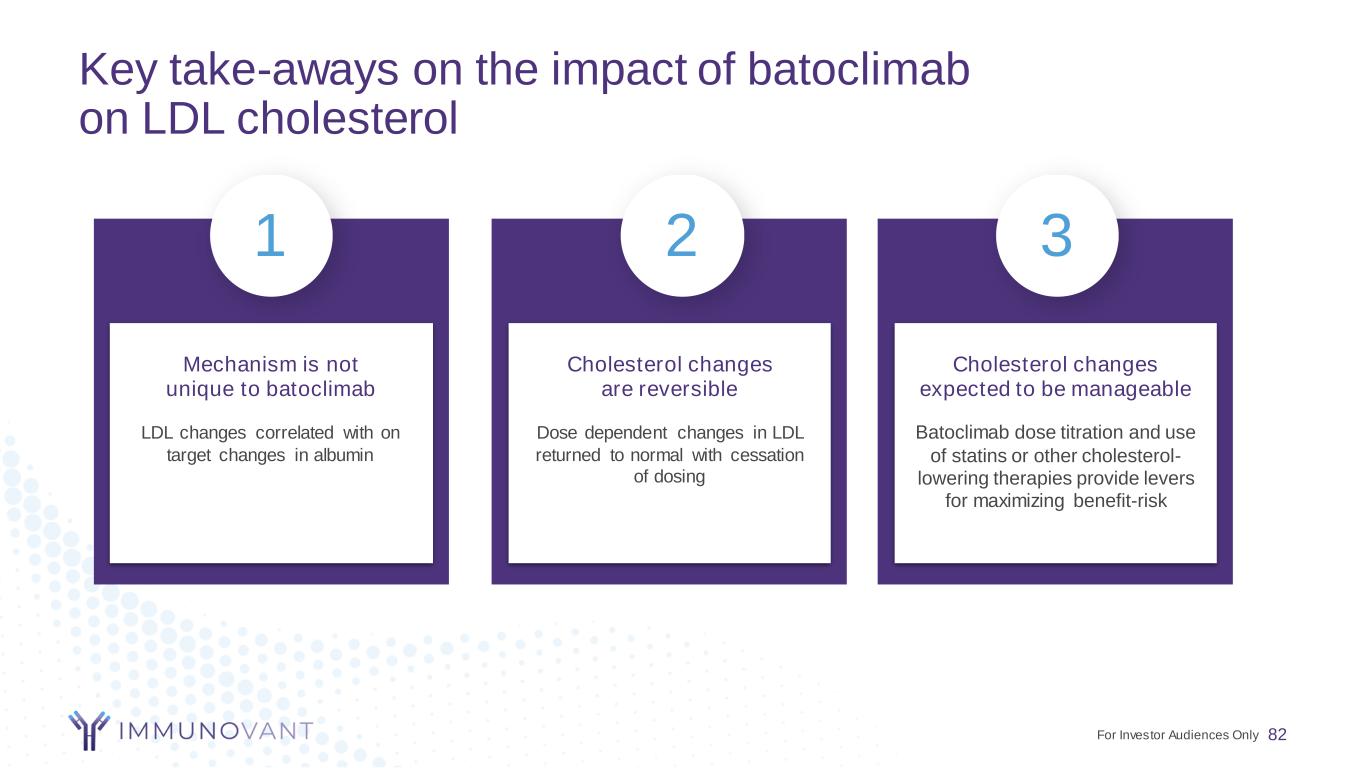
Key take-aways on the impact of batoclimab on LDL cholesterol 82 Mechanism is not unique to batoclimab Cholesterol changes are reversible Cholesterol changes expected to be manageable 1 2 3 LDL changes correlated with on target changes in albumin Dose dependent changes in LDL returned to normal with cessation of dosing Batoclimab dose titration and use of statins or other cholesterol- lowering therapies provide levers for maximizing benefit-risk For Investor Audiences Only

83

Path forward for Immunovant Pete Salzmann, MD Chief Executive Officer What comes next

Broad development plan enabled by an exciting mechanism of action, batoclimab’s features and a strong balance sheet 85 01 02 03 Immunovant remains on track to start three pivotal studies in calendar year 20223, and to announce two new indications by August 2022 1. As of December 31, 2021, per most recent Quarterly Report on Form 10-Q filed w ith the SEC on February 4, 2022 2. The assumptions upon w hich we have based our estimates are routinely evaluated and may be subject to change 3. Three pivotal trials include myasthenia gravis and tw o other indications Batoclimab's unique combination of attributes present a potentially significant commercial opportunity across multiple indications in rare autoimmune diseases Immunovant is well-capitalized with $527M1 in cash expected to fund its broad development plans into calendar year 20252 85 04 Immunovant plans to initiate a Phase 3 study of batoclimab in Myasthenia Gravis in the first half of calendar year 2022 – topline results expected in 2024 For Investor Audiences Only

Pete Salzmann, MD Chief Executive Officer Renee Barnett Chief Financial Officer Bill Macias Chief Medical Officer Investor Q&A