
For Investor Audiences Only Roivant R&D Day Investor Presentation September 28, 2021 Exhibit 99.2

For Investor Audiences Only Forward-looking statements 2 This presentation contains forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. The use of words such as “may,” “might,” “will,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “intend,” “future,” “potential,” “continue” and other similar expressions (as well as other words or expressions referencing future events, conditions, or circumstances) are intended to identify forward-looking statements. For example, forward-looking statements include statements Immunovant makes regarding its business strategy, its plans to develop and commercialize its product candidates, the potential safety and efficacy of Immunovant’s current or future product candidates, including batoclimab for Myasthenia Gravis, Thyroid Eye Disease and Warm Autoimmune Hemolytic Anemia, its expectations regarding timing, the design and results of clinical trials of its product candidates, Immunovant’s plans and expected timing with respect to regulatory filings and approvals, the size and growth potential of the markets for Immunovant’s product candidates, and its ability to serve those markets. All forward-looking statements are based on estimates and assumptions by Immunovant’s management that, although Immunovant believes to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those expressed or implied by such forward-looking statements. Such risks and uncertainties include, among others: initial results or other preliminary analyses or results of early clinical trials may not be predictive final trial results or of the results of later clinical trials; the timing and availability of data from clinical trials; the timing of discussions with regulatory agencies, as well as regulatory submissions and potential approvals; the continued development of Immunovant’s product candidates, including the timing of the commencement of additional clinical trials and resumption of current trials; Immunovant’s scientific approach, clinical trial design, indication selection and general development progress; future clinical trials may not confirm any safety, potency or other product characteristics described or assumed in this presentation; any product candidates that Immunovant develops may not progress through clinical development or receive required regulatory approvals within expected timelines or at all; Immunovant’s product candidates may not be beneficial to patients, or even if approved by regulatory authorities, successfully commercialized; the potential impact of the ongoing COVID-19 pandemic on Immunovant’s clinical development plans and timelines; Immunovant’s business is heavily dependent on the successful development, regulatory approval and commercialization of its sole product candidate, IMVT-1401; Immunovant is at an early stage in development of IMVT-1401; and Immunovant will require additional capital to fund its operations and advance IMVT-1401 through clinical development. These and other risks and uncertainties are more fully described in Immunovant’s periodic and other reports filed with the Securities and Exchange Commission (SEC), including in the section titled “Risk Factors” in Immunovant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2021 filed with the SEC on August 9, 2021. Any forward-looking statement speaks only as of the date on which it was made. Immunovant undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise.
For Investor Audiences Only Rethinking possibilities in autoimmune disease 3 Love Trailblazing All Voices Bolder Faster Our vision: Normal lives for people with autoimmune diseases
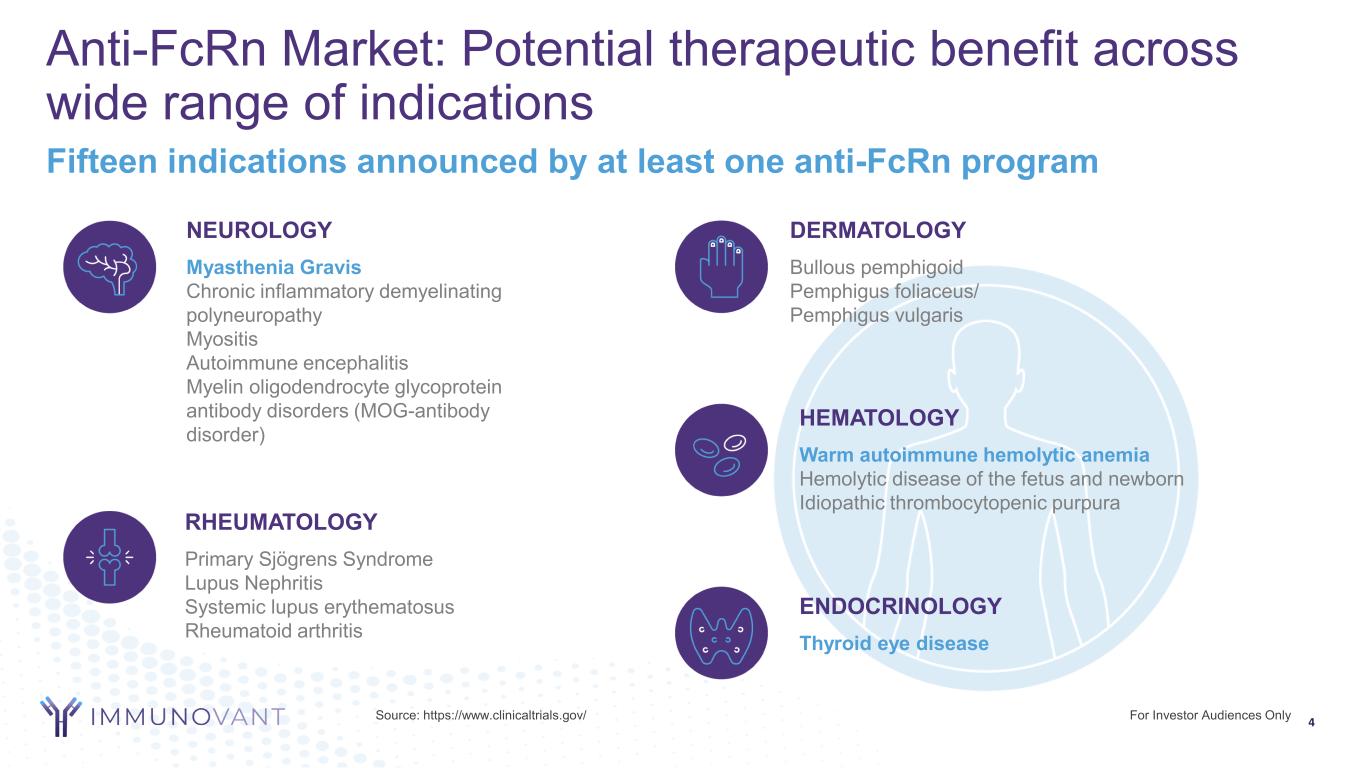
For Investor Audiences Only 4 Anti-FcRn Market: Potential therapeutic benefit across wide range of indications Fifteen indications announced by at least one anti-FcRn program ENDOCRINOLOGY Thyroid eye disease RHEUMATOLOGY Primary Sjögrens Syndrome Lupus Nephritis Systemic lupus erythematosus Rheumatoid arthritis DERMATOLOGY Bullous pemphigoid Pemphigus foliaceus/ Pemphigus vulgaris NEUROLOGY Myasthenia Gravis Chronic inflammatory demyelinating polyneuropathy Myositis Autoimmune encephalitis Myelin oligodendrocyte glycoprotein antibody disorders (MOG-antibody disorder) HEMATOLOGY Warm autoimmune hemolytic anemia Hemolytic disease of the fetus and newborn Idiopathic thrombocytopenic purpura Source: https://www.clinicaltrials.gov/
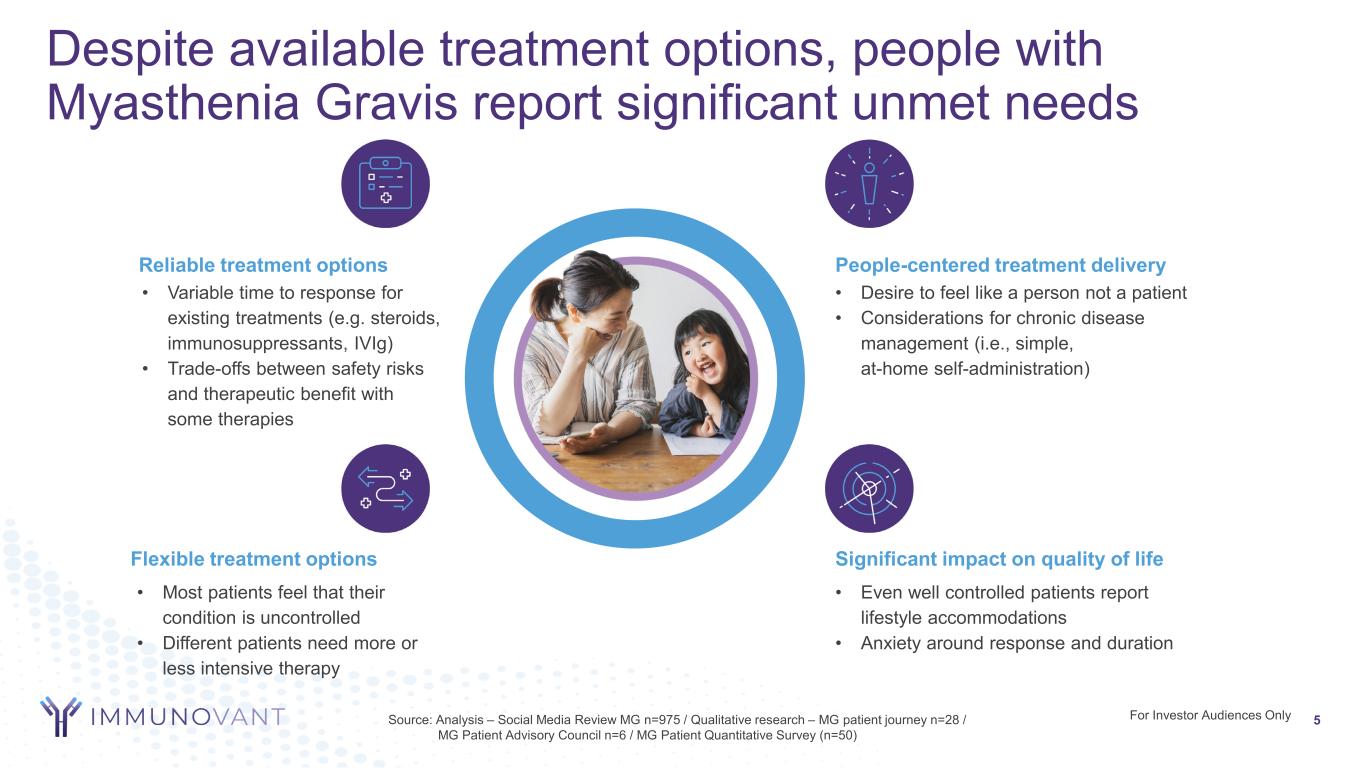
For Investor Audiences Only 5Source: Analysis – Social Media Review MG n=975 / Qualitative research – MG patient journey n=28 / MG Patient Advisory Council n=6 / MG Patient Quantitative Survey (n=50) • Even well controlled patients report lifestyle accommodations • Anxiety around response and duration Significant impact on quality of life • Variable time to response for existing treatments (e.g. steroids, immunosuppressants, IVIg) • Trade-offs between safety risks and therapeutic benefit with some therapies Reliable treatment options • Most patients feel that their condition is uncontrolled • Different patients need more or less intensive therapy Flexible treatment options • Desire to feel like a person not a patient • Considerations for chronic disease management (i.e., simple, at-home self-administration) People-centered treatment delivery Despite available treatment options, people with Myasthenia Gravis report significant unmet needs
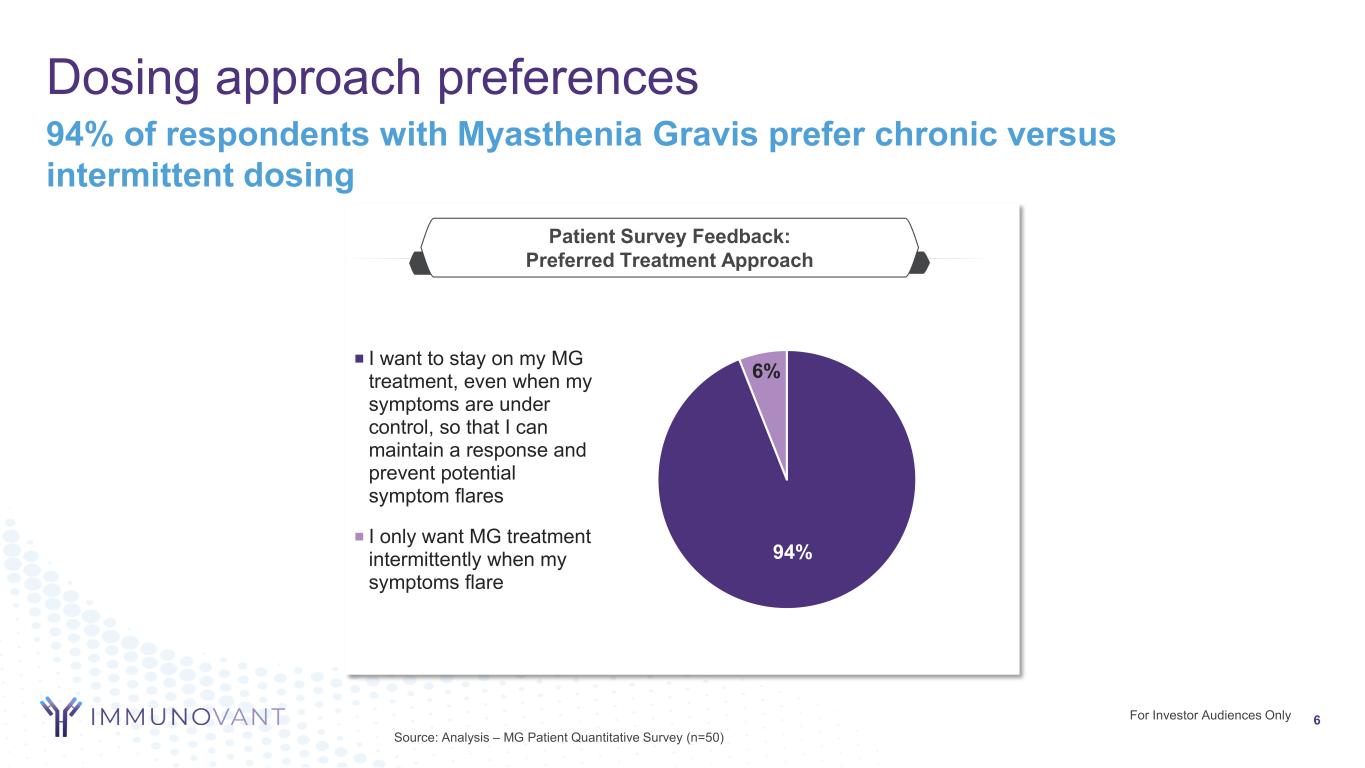
For Investor Audiences Only Dosing approach preferences 6 Source: Analysis – MG Patient Quantitative Survey (n=50) 94% of respondents with Myasthenia Gravis prefer chronic versus intermittent dosing Patient Survey Feedback: Preferred Treatment Approach 94% 6%I want to stay on my MG treatment, even when my symptoms are under control, so that I can maintain a response and prevent potential symptom flares I only want MG treatment intermittently when my symptoms flare
For Investor Audiences Only Batoclimab’s (IMVT-1401) differentiated attributes provide a unique opportunity to address patients’ unmet needs 7 Subcutaneous route of administration: Designed and developed for simple subcutaneous injection to provide human-centric, give and go dosing experience Significant impact on quality of life Reliable treatment options Flexible treatment options People-centered delivery of treatment Flexible dosing potential: Deep, rapid IgG suppression in the short-term; adjustable IgG suppression in the long-term Batoclimabli