Exhibit 99.1

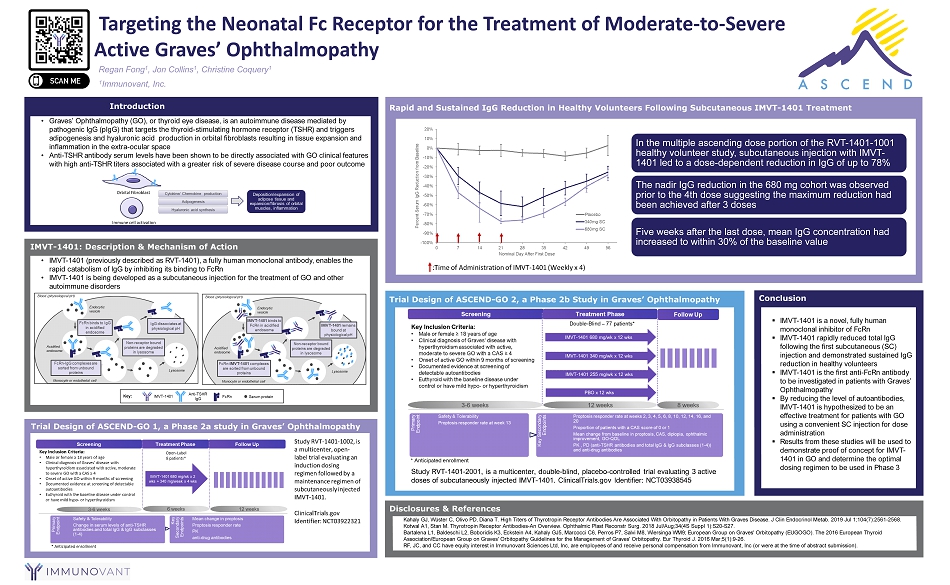
• Graves’ Ophthalmopathy (GO), or thyroid eye disease, is an autoimmune disease mediated by pathogenic IgG ( pIgG ) that targets the thyroid - stimulating hormone receptor (TSHR) and triggers adipogenesis and hyaluronic acid production in orbital fibroblasts resulting in tissue expansion and inflammation in the extra - ocular space • Anti - TSHR antibody serum levels have been shown to be directly associated with GO clinical features with high anti - TSHR titers associated with a greater risk of severe disease course and poor outcome • IMVT - 1401 (previously described as RVT - 1401), a fully human monoclonal antibody, enables the rapid catabolism of IgG by inhibiting its binding to FcRn • IMVT - 1401 is being developed as a subcutaneous injection for the treatment of GO and other autoimmune disorders ▪ IMVT - 1401 is a novel, fully human monoclonal inhibitor of FcRn ▪ IMVT - 1401 rapidly reduced total IgG following the first subcutaneous (SC) injection and demonstrated sustained IgG reduction in healthy volunteers ▪ IMVT - 1401 is the first anti - FcRn antibody to be investigated in patients with Graves’ Ophthalmopathy ▪ By reducing the level of autoantibodies, IMVT - 1401 is hypothesized to be an effective treatment for patients with GO using a convenient SC injection for dose administration ▪ Results from these studies will be used to demonstrate proof of concept for IMVT - 1401 in GO and determine the optimal dosing regimen to be used in Phase 3 IMVT - 1401: Description & Mechanism of Action Trial Design of ASCEND - GO 1, a Phase 2a study in Graves’ Ophthalmopathy Rapid and Sustained IgG Reduction in Healthy Volunteers Following Subcutaneous IMVT - 1401 Treatment Trial Design of ASCEND - GO 2, a Phase 2b Study in Graves’ Ophthalmopathy Conclusion Introduction Regan Fong 1 , Jon Collins 1 , Christine Coquery 1 Disclosures & References Kahaly GJ, Wüster C, Olivo PD, Diana T. High Titers of Thyrotropin Receptor Antibodies Are Associated With Orbitopathy in Patients With Graves Disease. J Clin Endocr ino l Metab . 2019 Jul 1;104(7):2561 - 2568. Kotwal A1, Stan M. Thyrotropin Receptor Antibodies - An Overview. Ophthalmic Plast Reconstr Surg. 2018 Jul/Aug;34(4S Suppl 1):S20 - S27. Bartalena L1, Baldeschi L2, Boboridis K3, Eckstein A4, Kahaly GJ5, Marcocci C6, Perros P7, Salvi M8, Wiersinga WM9; European Group on Graves' Orbitopathy (EUGOGO). The 2016 European Thyroid Association/European Group on Graves' Orbitopathy Guidelines for the Management of Graves' Orbitopathy. Eur Thyroid J. 2016 M ar; 5(1):9 - 26. RF, JC, and CC have equity interest in Immunovant Sciences Ltd, Inc, are employees of and receive personal compensation from Immunovant , Inc (or were at the time of abstract submission). Targeting the Neonatal Fc Receptor for the Treatment of Moderate - to - Severe Active Graves’ Ophthalmopathy 1 Immunovant, Inc. In the multiple ascending dose portion of the RVT - 1401 - 1001 healthy volunteer study, subcutaneous injection with IMVT - 1401 led to a dose - dependent reduction in IgG of up to 78% The nadir IgG reduction in the 680 mg cohort was observed prior to the 4th dose suggesting the maximum reduction had been achieved after 3 doses Five weeks after the last dose, mean IgG concentration had increased to within 30% of the baseline value