

ASCEND MG Topline Results Conference Call August 25, 2020 Exhibit 99.2

Forward-looking statements This presentation contains forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. The use of words such as “may,” “might,” “will,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “intend,” “future,” “potential,” “continue” and other similar expressions (as well as other words or expressions referencing future events, conditions, or circumstances) are intended to identify forward-looking statements. For example, forward-looking statements include statements Immunovant makes regarding its business strategy, its plans to develop and commercialize its product candidates, the potential safety and efficacy of Immunovant’s current or future product candidates, its expectations regarding timing, the design and results of clinical trials of its product candidates, Immunovant’s plans and expected timing with respect to regulatory filings and approvals, the size and growth potential of the markets for Immunovant’s product candidates, and its ability to serve those markets. All forward-looking statements are based on estimates and assumptions by Immunovant’s management that, although Immunovant believes to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those expressed or implied by such forward-looking statements. Such risks and uncertainties include, among others: initial results or other preliminary analyses or results of early clinical trials may not be predictive final trial results or of the results of later clinical trials; future clinical trials may not confirm any safety, potency or other product characteristics described or assumed in this presentation; any product candidates that Immunovant develops may not progress through clinical development or receive required regulatory approvals within expected timelines or at all; Immunovant’s product candidates may not be beneficial to patients, or even if approved by regulatory authorities, successfully commercialized; the potential impact of the ongoing COVID-19 pandemic on Immunovant’s clinical development plans and timelines; Immunovant’s business is heavily dependent on the successful development, regulatory approval and commercialization of its sole product candidate, IMVT-1401; Immunovant is at an early stage in development of IMVT-1401; and Immunovant will require additional capital to fund its operations and advance IMVT-1401 through clinical development. These and other risks and uncertainties are more fully described in Immunovant’s periodic and other reports filed with the Securities and Exchange Commission (SEC), including in the section titled “Risk Factors” in Immunovant’s most recent Quarterly Report on Form 10-Q filed with the SEC on August 12, 2020. Any forward-looking statement speaks only as of the date on which it was made. Immunovant undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise.
ASCEND MG topline results are extremely encouraging for patients suffering from Myasthenia Gravis Only subcutaneous injection anti-FcRn agent in clinical development for MG Safety and tolerability findings similar to prior IMVT-1401 studies Robust, dose-dependent, IgG reductions Statistically significant improvements in MG-ADL and MGC scales High rate of deep responders across MG scales
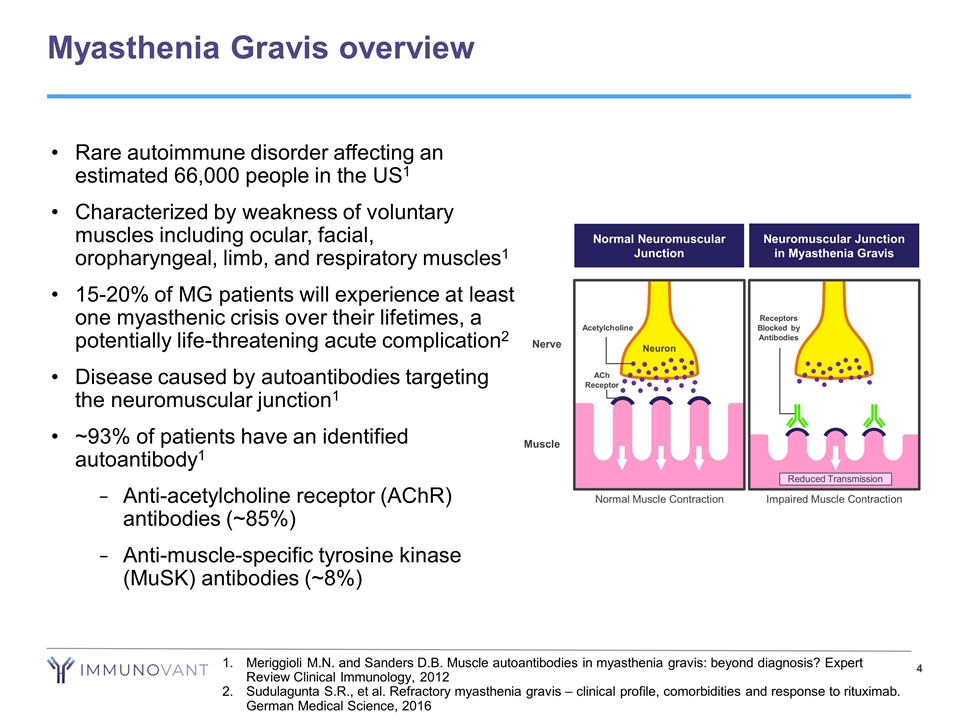
Myasthenia Gravis overview Meriggioli M.N. and Sanders D.B. Muscle autoantibodies in myasthenia gravis: beyond diagnosis? Expert Review Clinical Immunology, 2012 Sudulagunta S.R., et al. Refractory myasthenia gravis – clinical profile, comorbidities and response to rituximab. German Medical Science, 2016 Rare autoimmune disorder affecting an estimated 66,000 people in the US1 Characterized by weakness of voluntary muscles including ocular, facial, oropharyngeal, limb, and respiratory muscles1 15-20% of MG patients will experience at least one myasthenic crisis over their lifetimes, a potentially life-threatening acute complication2 Disease caused by autoantibodies targeting the neuromuscular junction1 ~93% of patients have an identified autoantibody1 Anti-acetylcholine receptor (AChR) antibodies (~85%) Anti-muscle-specific tyrosine kinase (MuSK) antibodies (~8%) Muscle Neuromuscular Junction in Myasthenia Gravis Reduced Transmission Impaired Muscle Contraction Receptors Blocked by Antibodies Neuron Nerve Acetylcholine ACh Receptor Normal Muscle Contraction Normal Neuromuscular Junction
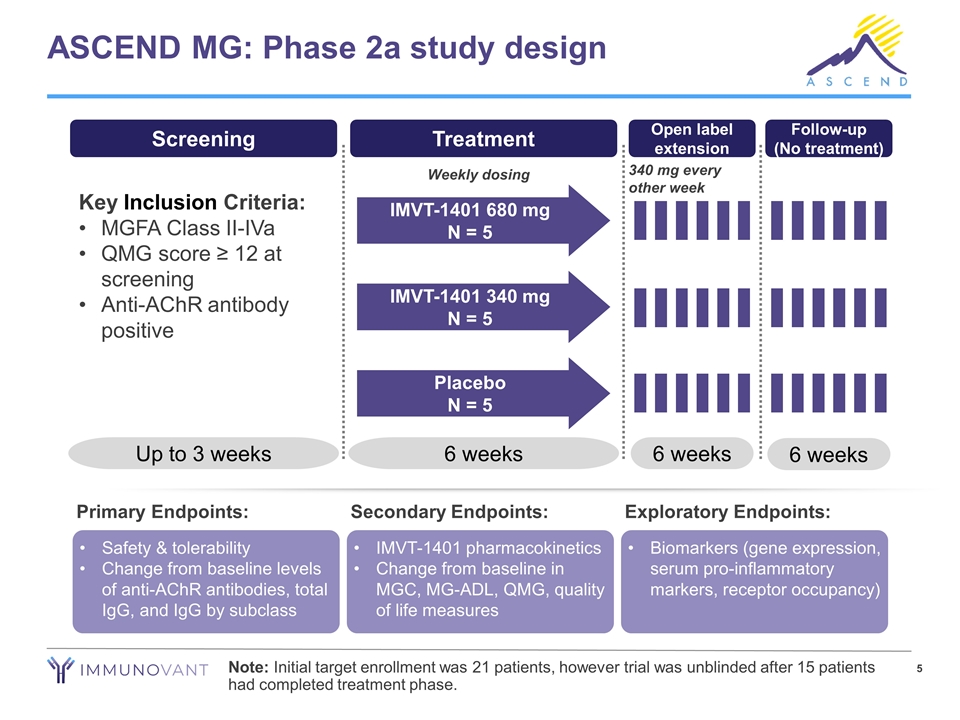
ASCEND MG: Phase 2a study design Safety & tolerability Change from baseline levels of anti-AChR antibodies, total IgG, and IgG by subclass Primary Endpoints: IMVT-1401 pharmacokinetics Change from baseline in MGC, MG-ADL, QMG, quality of life measures Secondary Endpoints: Biomarkers (gene expression, serum pro-inflammatory markers, receptor occupancy) Exploratory Endpoints: Screening Key Inclusion Criteria: MGFA Class II-IVa QMG score ≥ 12 at screening Anti-AChR antibody positive Up to 3 weeks Treatment 6 weeks IMVT-1401 680 mg N = 5 IMVT-1401 340 mg N = 5 Placebo N = 5 6 weeks Follow-up (No treatment) Open label extension 6 weeks 340 mg every other week Note: Initial target enrollment was 21 patients, however trial was unblinded after 15 patients had completed treatment phase. Weekly dosing
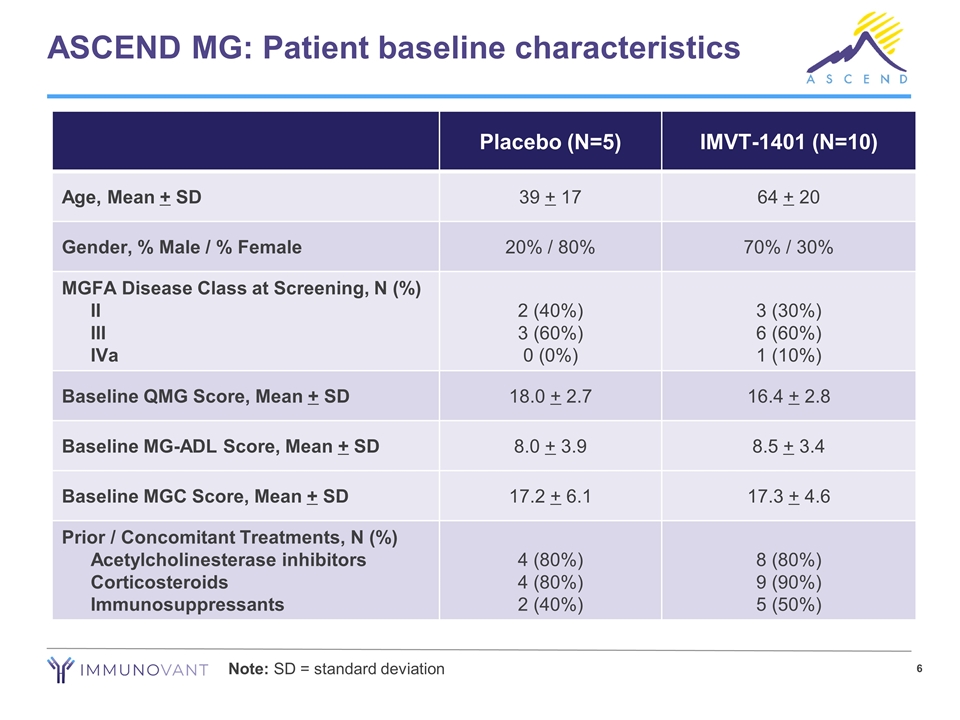
ASCEND MG: Patient baseline characteristics Note: SD = standard deviation Placebo (N=5) IMVT-1401 (N=10) Age, Mean + SD 39 + 17 64 + 20 Gender, % Male / % Female 20% / 80% 70% / 30% MGFA Disease Class at Screening, N (%) II III IVa 2 (40%) 3 (60%) 0 (0%) 3 (30%) 6 (60%) 1 (10%) Baseline QMG Score, Mean + SD 18.0 + 2.7 16.4 + 2.8 Baseline MG-ADL Score, Mean + SD 8.0 + 3.9 8.5 + 3.4 Baseline MGC Score, Mean + SD 17.2 + 6.1 17.3 + 4.6 Prior / Concomitant Treatments, N (%) Acetylcholinesterase inhibitors Corticosteroids Immunosuppressants 4 (80%) 4 (80%) 2 (40%) 8 (80%) 9 (90%) 5 (50%)
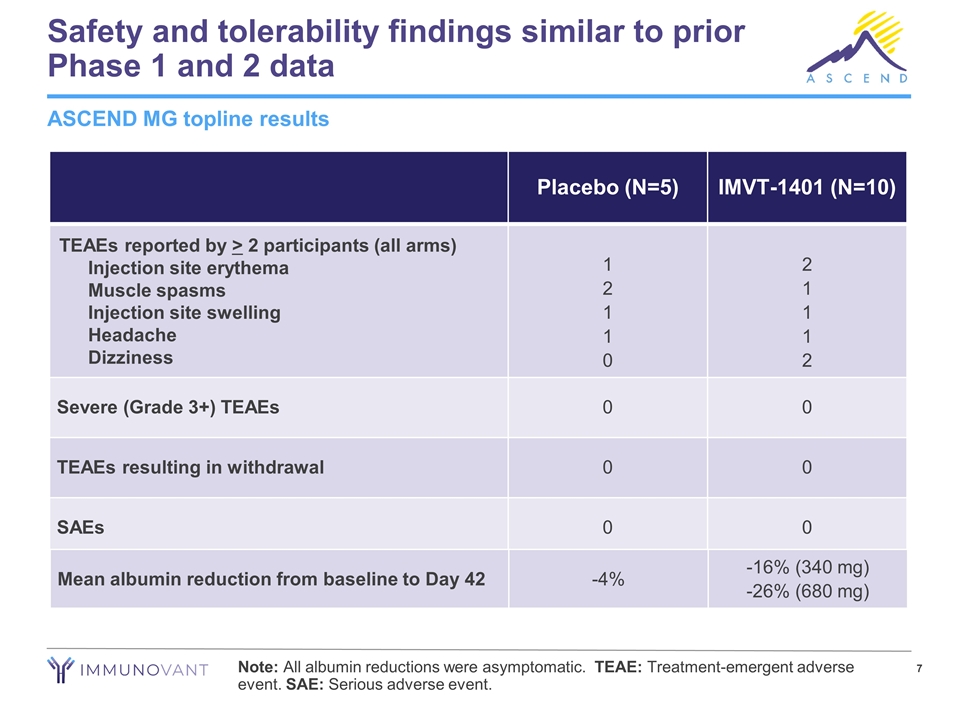
Safety and tolerability findings similar to prior Phase 1 and 2 data Placebo (N=5) IMVT-1401 (N=10) TEAEs reported by > 2 participants (all arms) Injection site erythema Muscle spasms Injection site swelling Headache Dizziness 1 2 1 1 0 2 1 1 1 2 Severe (Grade 3+) TEAEs 0 0 TEAEs resulting in withdrawal 0 0 SAEs 0 0 ASCEND MG topline results Mean albumin reduction from baseline to Day 42 -4% -16% (340 mg) -26% (680 mg) Note: All albumin reductions were asymptomatic. TEAE: Treatment-emergent adverse event. SAE: Serious adverse event.
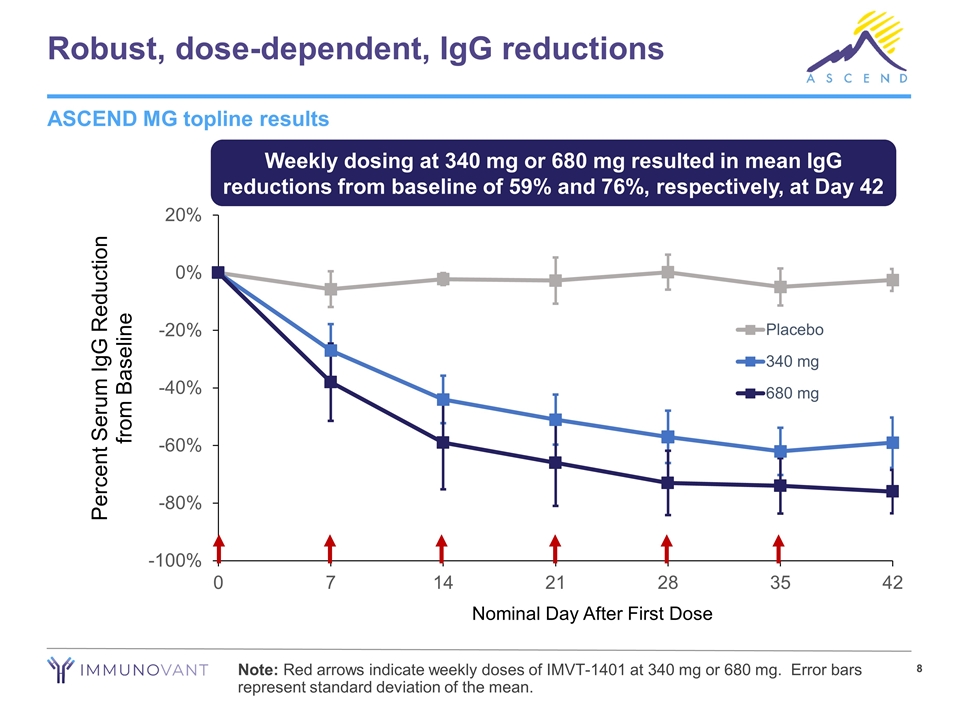
Robust, dose-dependent, IgG reductions Weekly dosing at 340 mg or 680 mg resulted in mean IgG reductions from baseline of 59% and 76%, respectively, at Day 42 ASCEND MG topline results Note: Red arrows indicate weekly doses of IMVT-1401 at 340 mg or 680 mg. Error bars represent standard deviation of the mean.
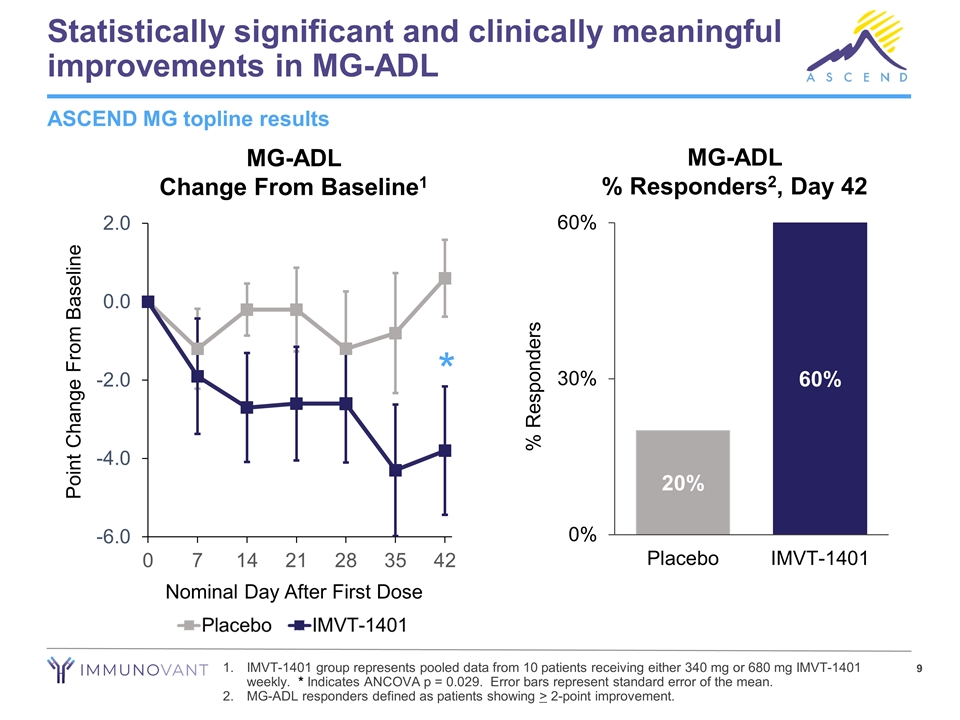
Statistically significant and clinically meaningful improvements in MG-ADL IMVT-1401 group represents pooled data from 10 patients receiving either 340 mg or 680 mg IMVT-1401 weekly. * Indicates ANCOVA p = 0.029. Error bars represent standard error of the mean. MG-ADL responders defined as patients showing > 2-point improvement. ASCEND MG topline results * MG-ADL Change From Baseline1 MG-ADL % Responders2, Day 42

Overview of clinical efficacy measures utilized as endpoints in ASCEND MG Myasthenia Gravis Activities of Daily Living (MG-ADL): Patient-reported questionnaire Comprised of 8 items reflecting ocular, bulbar, respiratory, and limb symptoms and their impact on function Validated FDA regulatory endpoint Quantitative Myasthenia Gravis (QMG): Physician assessment Comprised of 13 items reflecting ocular symptoms, facial/oropharyngeal symptoms, and nonfacial symptoms Lung function test challenging to administer during pandemic Myasthenia Gravis Composite (MGC): Combination patient and physician assessment 10 test items selected using the following criteria: Meaningful to both the physician and the patient Frequently abnormal in patients with active disease Responsive to clinical change Currently endorsed by the Myasthenia Gravis Foundation of America (MGFA) as the scale of choice for prospective studies in MG
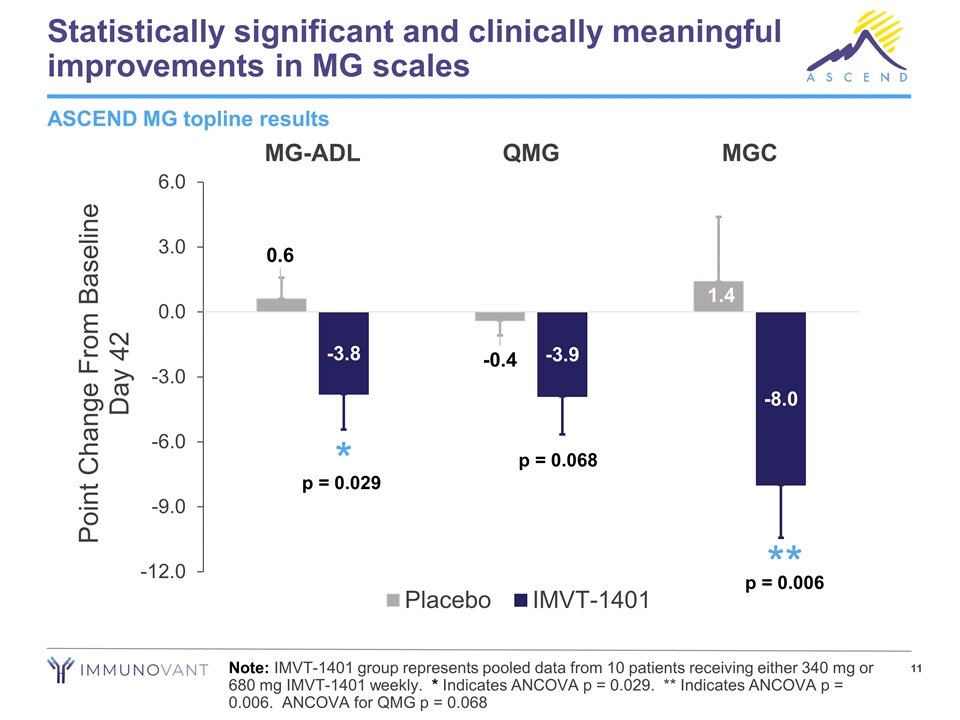
Statistically significant and clinically meaningful improvements in MG scales Note: IMVT-1401 group represents pooled data from 10 patients receiving either 340 mg or 680 mg IMVT-1401 weekly. * Indicates ANCOVA p = 0.029. ** Indicates ANCOVA p = 0.006. ANCOVA for QMG p = 0.068 ASCEND MG topline results * ** p = 0.029 p = 0.006 p = 0.068
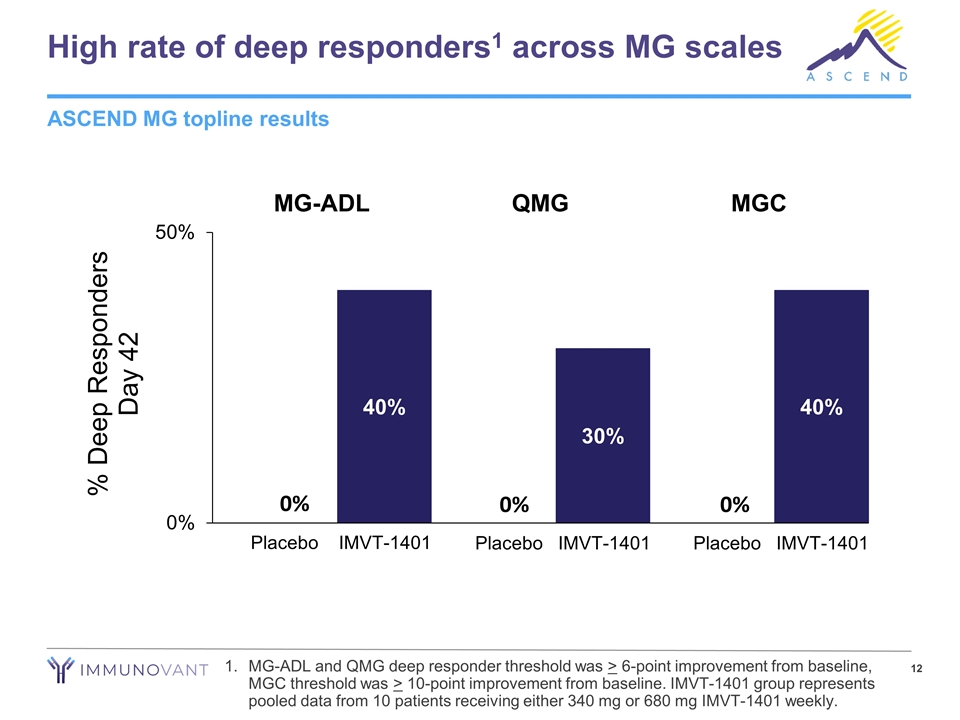
High rate of deep responders1 across MG scales MG-ADL and QMG deep responder threshold was > 6-point improvement from baseline, MGC threshold was > 10-point improvement from baseline. IMVT-1401 group represents pooled data from 10 patients receiving either 340 mg or 680 mg IMVT-1401 weekly. ASCEND MG topline results 0% 0% 0%
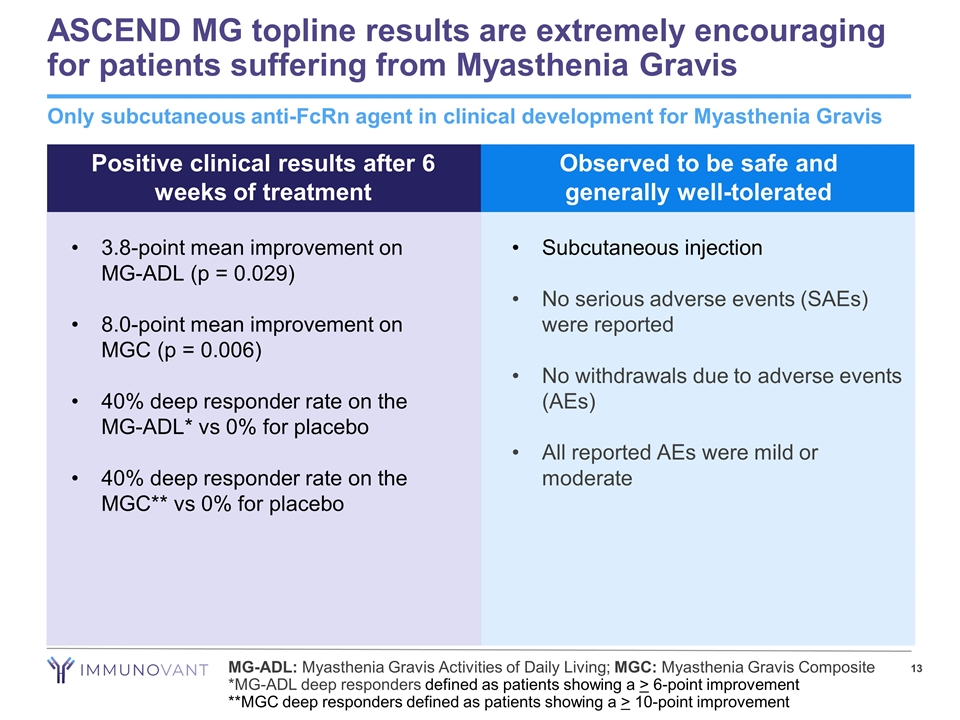
ASCEND MG topline results are extremely encouraging for patients suffering from Myasthenia Gravis Only subcutaneous anti-FcRn agent in clinical development for Myasthenia Gravis Positive clinical results after 6 weeks of treatment Observed to be safe and generally well-tolerated Subcutaneous injection No serious adverse events (SAEs) were reported No withdrawals due to adverse events (AEs) All reported AEs were mild or moderate 3.8-point mean improvement on MG-ADL (p = 0.029) 8.0-point mean improvement on MGC (p = 0.006) 40% deep responder rate on the MG-ADL* vs 0% for placebo 40% deep responder rate on the MGC** vs 0% for placebo MG-ADL: Myasthenia Gravis Activities of Daily Living; MGC: Myasthenia Gravis Composite *MG-ADL deep responders defined as patients showing a > 6-point improvement **MGC deep responders defined as patients showing a > 10-point improvement
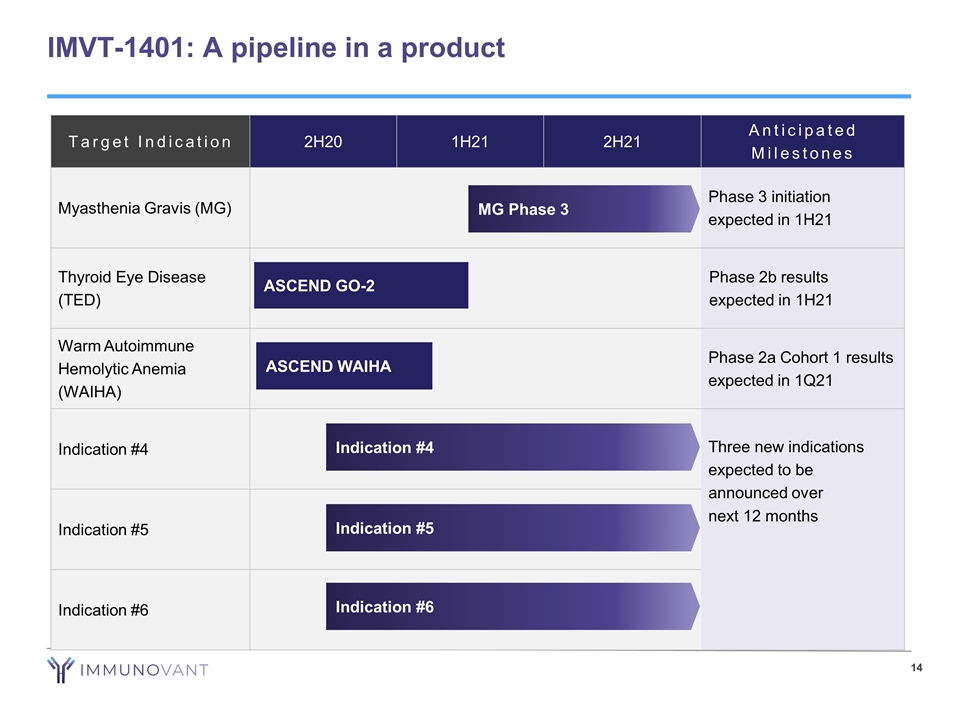
IMVT-1401: A pipeline in a product Target Indication 2H20 1H21 2H21 Anticipated Milestones Myasthenia Gravis (MG) Phase 3 initiation expected in 1H21 Thyroid Eye Disease (TED) Phase 2b results expected in 1H21 Warm Autoimmune Hemolytic Anemia (WAIHA) Phase 2a Cohort 1 results expected in 1Q21 Indication #4 Three new indications expected to be announced over next 12 months Indication #5 Indication #6 MG Phase 3 ASCEND GO-2 ASCEND WAIHA Indication #4 Indication #5 Indication #6

Question and answer session